In 2020, NIAID and its global partners rapidly leveraged their scientific infrastructure and expertise to conduct critical basic, preclinical, and clinical research to address the coronavirus disease 2019 (COVID-19) pandemic. Although considerable challenges remain, scientists have made enormous strides this year in understanding, preventing, and treating COVID-19. Other research brought us closer to a new option for HIV prevention, a shorter treatment regimen for drug-susceptible tuberculosis, and a potential new eczema treatment, among many other advances.
Ten selected science news highlights from 2020 are summarized below. All demonstrate how public investment in biomedical research drives scientific progress and benefits human health.
Investigational COVID-19 Vaccine Generates Immune Response in Older Adults
Results published in September from a Phase 1 trial of an investigational COVID-19 vaccine known as mRNA-1273 showed that the vaccine is well-tolerated and generates a strong immune response in older adults, who are more vulnerable to complications of COVID-19 and are an important population for vaccination. The mRNA vaccine candidate was co-developed by NIAID and the Massachusetts-based biotechnology company Moderna, Inc. According to the researchers, these results support testing of the investigational vaccine in older adults in an ongoing Phase 3 trial.
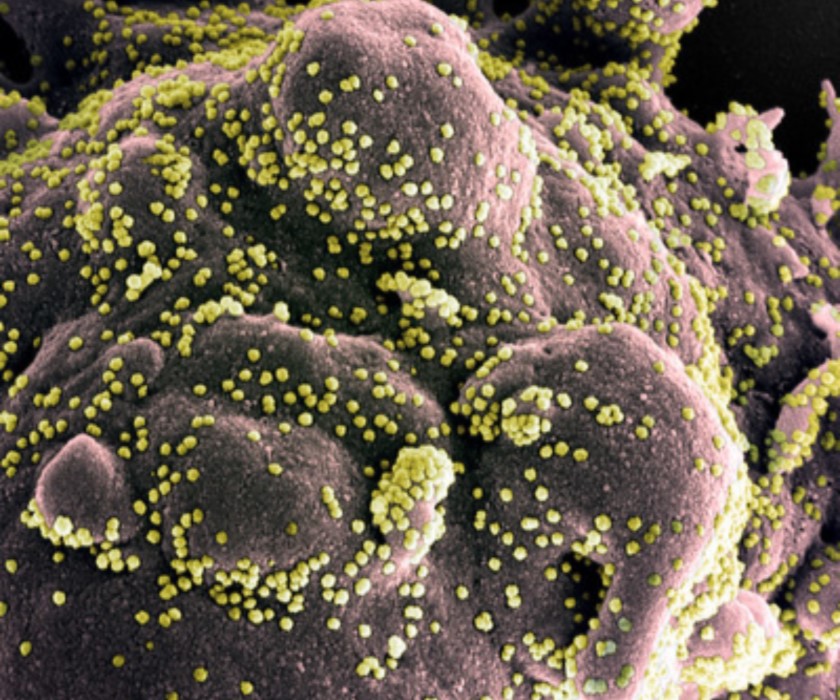
This colorized scanning electron micrograph shows SARS-CoV-2 particles (yellow) isolated from a patient.
Credit: NIAID

Long-Acting Injectable Drug Prevents HIV Acquisition
A pre-exposure prophylaxis (PrEP) regimen containing an investigational long-acting form of the HIV drug cabotegravir injected once every eight weeks was safe and more effective than daily oral PrEP at preventing HIV acquisition, two NIAID-sponsored clinical trials revealed. One trial enrolled more than 4,500 cisgender men and transgender women who have sex with men, and the companion study enrolled more than 3,200 cisgender women in southern and east Africa. These findings mark the first time a systemic, long-acting form of HIV prevention has shown to be highly effective in large-scale clinical trials.
Beaded pin with an HIV/AIDS awareness ribbon.
Credit: NIAID

Phase 3 Clinical Trials of Investigational COVID-19 Vaccines Begin
Safe and effective vaccines will be essential to meet the global need for widespread protection against COVID-19. NIAID is supporting several ongoing Phase 3 clinical trials assessing investigational vaccine candidates. The first of these launched in July to evaluate whether an investigational vaccine known as mRNA-1273 can prevent symptomatic COVID-19. In August, a trial evaluating an investigational COVID-19 vaccine known as AZD1222 began. Another trial, launched in September, is evaluating the investigational Janssen COVID-19 vaccine (JNJ-78436725).
Vial of investigational Janssen COVID-19 vaccine.
Credit: The Janssen Pharmaceutical Companies of Johnson & Johnson
COVID-19 Vaccine Shown Safe and Effective in NIH-Moderna Phase 3 Trial
In mid-November, an interim review of data from a Phase 3 trial of the investigational COVID-19 vaccine known as mRNA-1273 suggested that the vaccine is safe and effective at preventing symptomatic COVID-19 in adults. The interim analysis comprised 95 cases of symptomatic COVID-19 among volunteers. The independent data and safety monitoring board overseeing the trial reported that mRNA-1273 was safe and well-tolerated and noted a vaccine efficacy rate of 94.5%. On November 30, Moderna announced that analysis of data from 196 COVID-19 cases, of which 30 were severe, confirmed the high efficacy observed at the interim analysis. The updated overall vaccine efficacy was 94.1%, and efficacy against severe COVID-19 was 100%. Based on data from the trial, FDA granted Emergency Use Authorization for mRNA-1273 on December 18. The trial is ongoing.
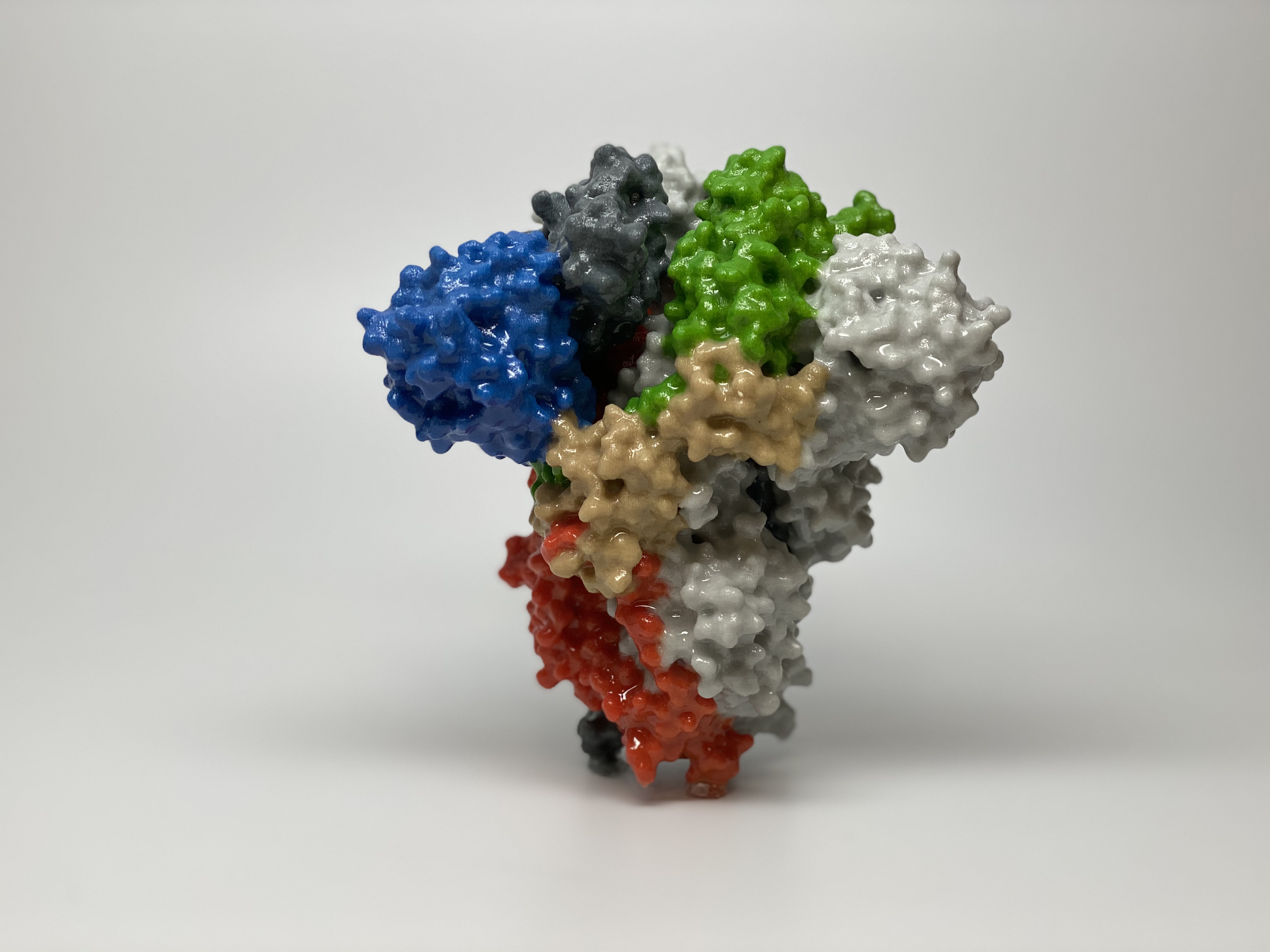
3D print of a spike protein on the surface of SARS-CoV-2, the virus that causes COVID-19. Spike proteins cover the surface of SARS-CoV-2 and enable the virus to enter and infect human cells.
Credit: NIH
NIH Launches Clinical Trials to Test Experimental Therapeutics for COVID-19
NIAID is supporting multiple clinical trials to evaluate potential new therapeutics for COVID-19 under NIH’s Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) program, a public-private partnership to speed development of the most promising treatments and vaccine candidates. This includes ACTIV-2 and ACTIV-3, both launched in August, and the ACTIV-5 Big Effect Trial (BET), which began in October. ACTIV-2 is evaluating the safety and efficacy of potential new therapeutics for COVID-19 among volunteers with mild to moderate COVID-19 not requiring hospitalization. The Phase 3 trial known as ACTIV-3 is designed to expand to test multiple different kinds of monoclonal antibody treatments among patients hospitalized with COVID-19. ACTIV-5/BET will determine whether certain approved therapies or investigational drugs in late-stage clinical development show promise against COVID-19 and merit advancement into larger clinical trials.
This colorized scanning electron micrograph shows SARS-CoV-2 particles (yellow) isolated from a patient.
Credit: NIAID
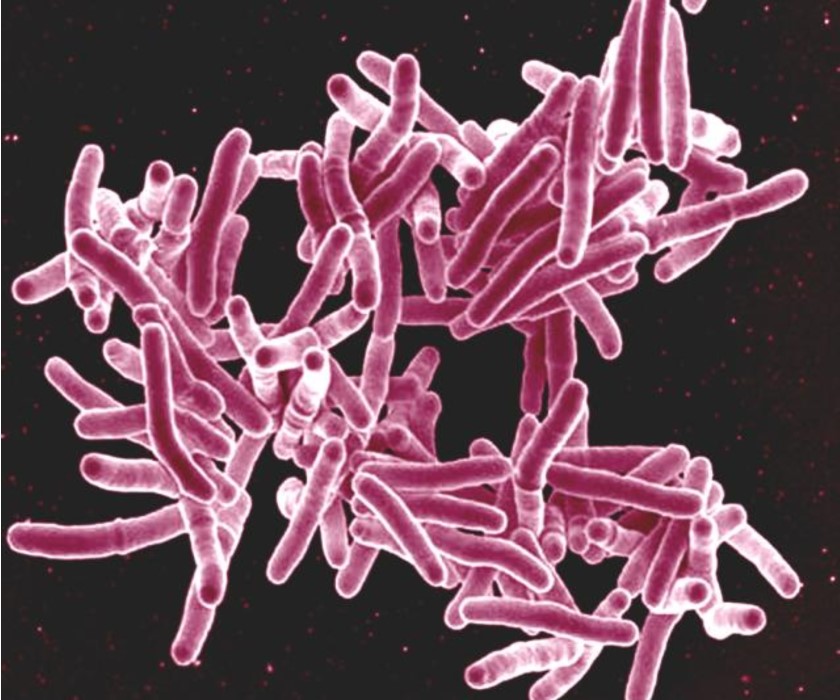
Landmark Study Identifies Short-Course TB Treatment
A new four-month daily treatment regimen is as safe and effective as the existing standard six-month regimen at curing drug-susceptible tuberculosis (TB) disease, according to results from a clinical trial led by CDC with collaboration from NIAID. Shortening treatment for TB disease enables patients to be cured faster, and has the potential to reduce treatment costs, improve patient quality of life, increase completion of therapy, and reduce development of drug resistance.
Scanning electron micrograph of Mycobacterium tuberculosis bacteria, which cause TB.
Credit: NIAID
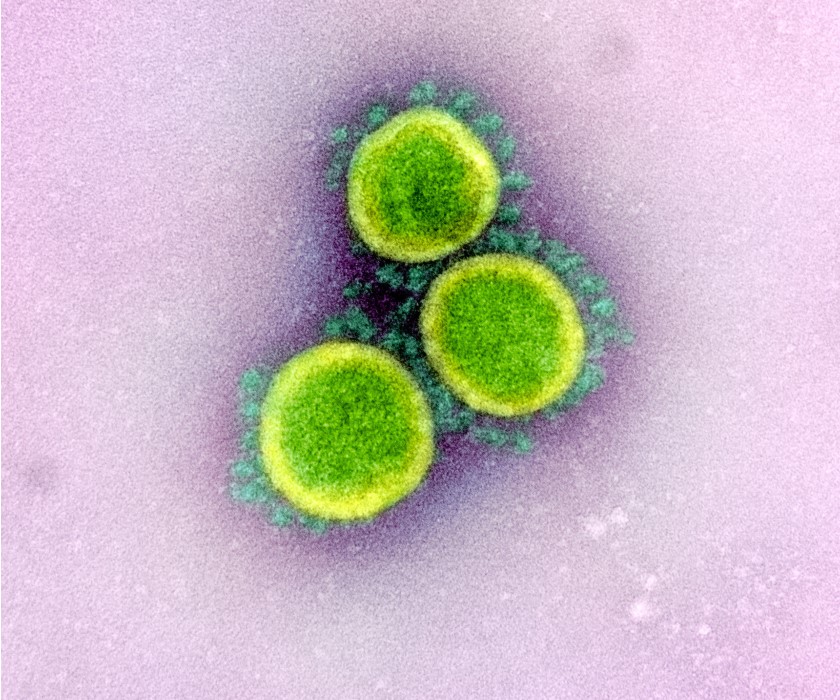
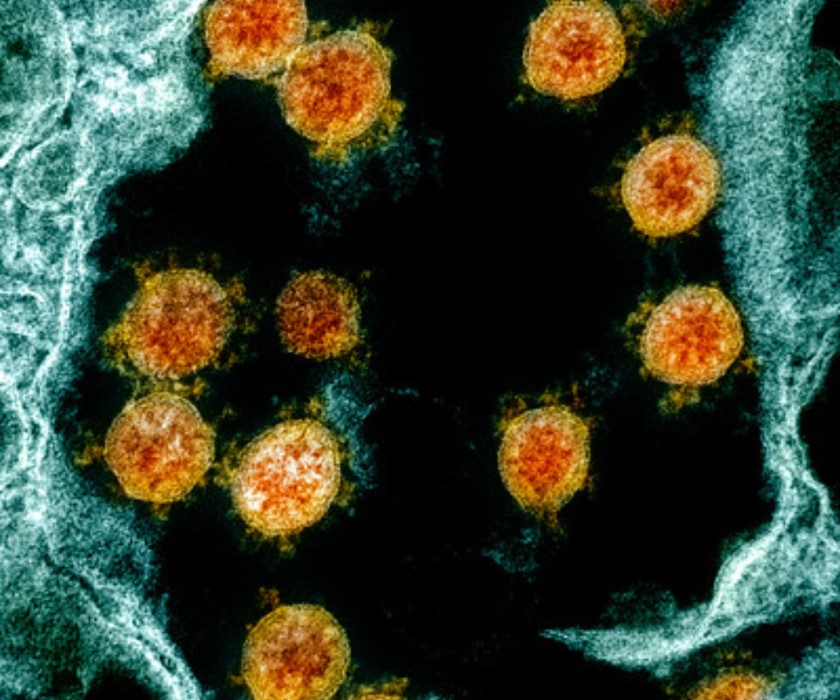
Study Assesses Incidence of Coronavirus Infection in Children
In May, NIAID announced that a study to help determine the rate of novel coronavirus infection in children and their family members in the United States had begun enrolling participants. The NIAID-sponsored study, called Human Epidemiology and Response to SARS-CoV-2 (HEROS), also will help determine the percentage of children infected with SARS-CoV-2, the virus that causes COVID-19, who develop symptoms of the disease. In addition, the ongoing study will examine whether rates of SARS-CoV-2 infection differ between children who have asthma or other allergic conditions and children who do not.
Transmission electron micrograph of SARS-CoV-2 particles isolated from a patient.
Credit: NIAID
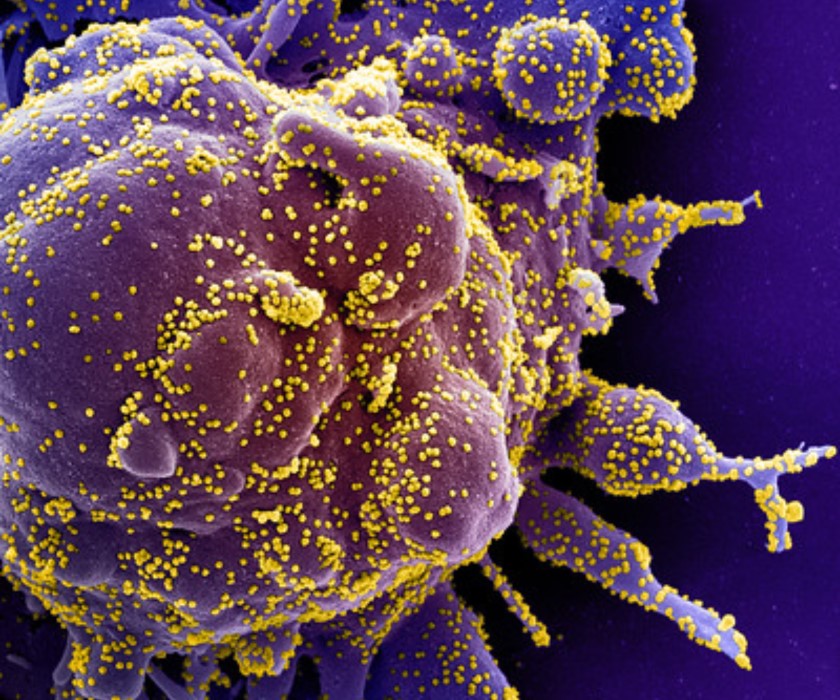
Remdesivir for COVID-19 Improves Time to Recovery
In May, investigators published a preliminary report from the Adaptive COVID-19 Treatment Trial (ACTT), which found that participants hospitalized with COVID-19 who received the antiviral drug remdesivir had a shorter time to recovery than those who received placebo. Subsequently, findings from ACTT-2 indicated that a combination of baricitinib and remdesivir shortened time to recovery relative to treatment with a placebo and remdesivir. ACTT-4 aims to determine whether baricitinib or the corticosteroid dexamethasone, when administered with remdesivir, is more effective at preventing hospitalized adults on supplemental oxygen from progressing to requiring mechanical ventilation or death, among other outcomes, or if they are similar. ACTT-3 is evaluating remdesivir plus the immunomodulator interferon beta-1a.
Colorized scanning electron micrograph of an apoptotic cell (purple) heavily infected with SARS-CoV-2 particles (yellow), isolated from a patient sample. Image captured at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland.
Credit: NIAID
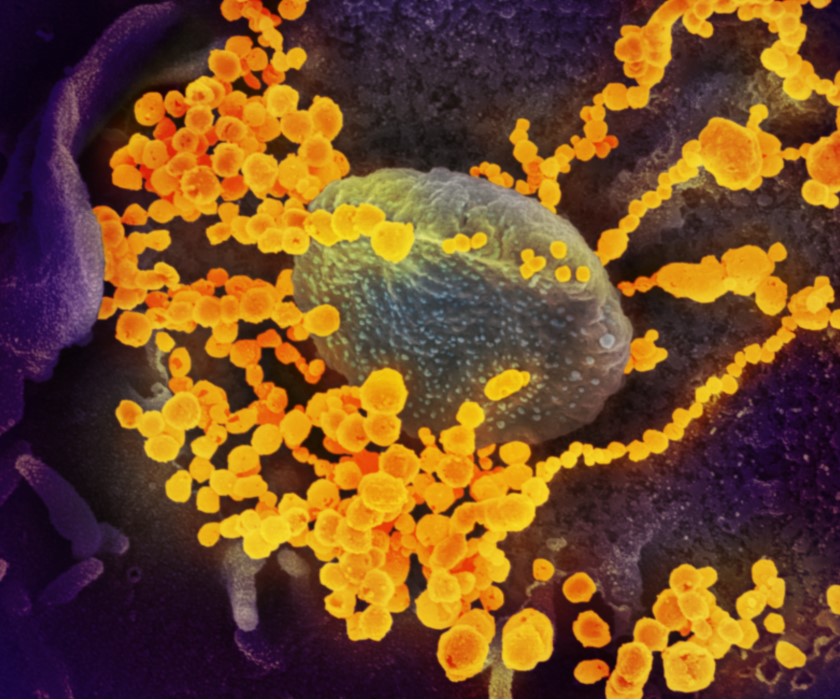
Animal Model Aids Development of COVID-19 Vaccines and Therapeutics
Scientists at NIAID’s Rocky Mountain Laboratories (RML) in Hamilton, Montana, developed a rhesus macaque model of mild- to-moderate human COVID-19 to aid development of vaccines and therapeutics. In April, they reported that early treatment with the experimental antiviral drug remdesivir significantly reduced clinical disease and damage to the lungs of monkeys infected with SARS-CoV-2, the virus that causes COVID-19. These results supported clinical testing of remdesivir in a large NIAID-sponsored trial. In May, the RML scientists and collaborators reported that a single dose of ChAdOx1 nCoV-19, an investigational vaccine against SARS-CoV-2 developed at the University of Oxford Jenner Institute, protected six rhesus macaques from pneumonia caused by the virus. Based on these data, a Phase 1 trial of the candidate vaccine began in healthy volunteers in the United Kingdom.
This scanning electron microscope image shows SARS-CoV-2 (round gold objects) emerging from the surface of cells cultured in the lab. SARS-CoV-2 is the virus that causes COVID-19. The virus shown was isolated from a patient in the U.S.
Credit: NIAID-RML
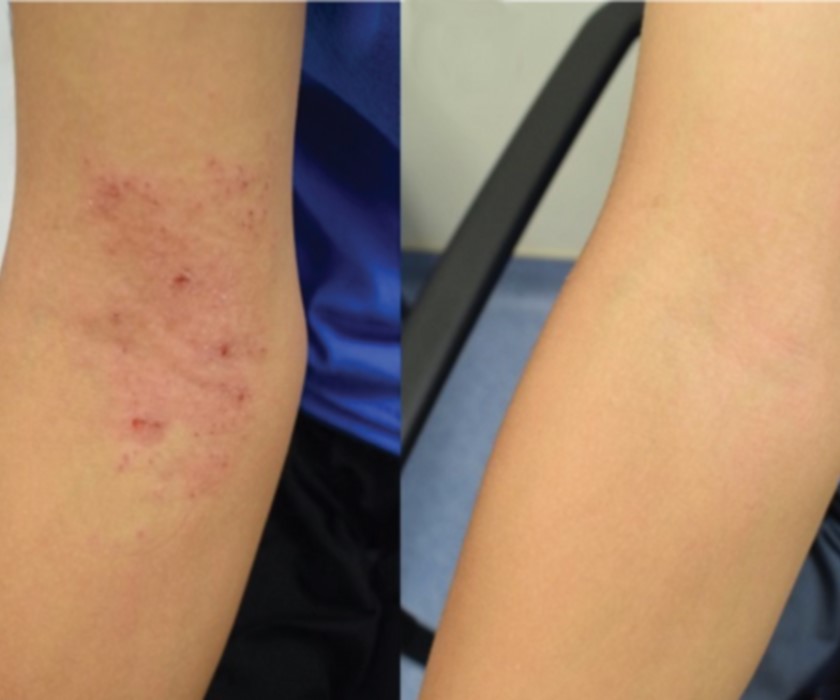
Probiotic Skin Therapy Improves Eczema in Children
An experimental treatment for eczema that aims to modify the skin microbiome safely reduced disease severity and increased quality of life for children as young as 3 years of age, an early-stage NIAID clinical found. These improvements persisted for up to eight months after treatment stopped. The investigational treatment is now being evaluated in a Phase 2 placebo-controlled trial.
Inner elbow of a child with eczema before receiving the experimental therapy (left) and after four months of treatment (right).
Credit: NIAID

