Alicia Widge M.D. is the Associate Chief for Clinical Research and Development in the VRC’s Clinical Trials Program. In this role, she serves as a Principal Investigator for multiple Phase 1, first-in-human clinical trials, including a COVID-19 mRNA vaccine, universal influenza vaccine candidates with novel adjuvants, a Nipah virus vaccine, and HIV and Ebola monoclonal antibodies. She is responsible for trial development, scientific guidance, industry collaborations, and leading Investigational new Drug (IND) sponsor responsibilities, including pharmacovigilance, monitoring oversight, clinical trials risk assessment, and quality management for the VRC’s Clinical Trials Program. She is also the clinical lead for collaborations with multiple academic and industry partners.
Principal Investigator
Associate Investigator
Coates EE, Edupuganti S, Chen GL, Happe M, Strom L, Widge A, Florez MB, Cox JH, Gordon I, Plummer S, Ola A, Yamshchikov G, Andrews C, Curate-Ingram S, Morgan P, Nagar S, Collins MH, Bray A, Nguyen T, Stein J, Case CL, Kaltovich F, Wycuff D, Liang CJ, Carlton K, Vazquez S, Mascola JR, Ledgerwood JE; VRC 313 Study Team. Safety and immunogenicity of a trivalent virus-like particle vaccine against western, eastern, and Venezuelan equine encephalitis viruses: a phase 1, open-label, dose-escalation, randomised clinical trial. Lancet Infect Dis. 2022 May 11:S1473-3099(22)00052-4.
Casazza JP, Cale EM, Narpala S, Yamshchikov GV, Coates EE, Hendel CS, Novik L, Holman LA, Widge AT, Apte P, Gordon I, Gaudinski MR, Conan-Cibotti M, Lin BC, Nason MC, Trofymenko O, Telscher S, Plummer SH, Wycuff D, Adams WC, Pandey JP, McDermott A, Roederer M, Sukienik AN, O'Dell S, Gall JG, Flach B, Terry TL, Choe M, Shi W, Chen X, Kaltovich F, Saunders KO, Stein JA, Doria-Rose NA, Schwartz RM, Balazs AB, Baltimore D, Nabel GJ, Koup RA, Graham BS, Ledgerwood JE, Mascola JR; VRC 603 Study Team. Safety and tolerability of AAV8 delivery of a broadly neutralizing antibody in adults living with HIV: a phase 1, dose-escalation trial. Nat Med. 2022 May;28(5):1022-1030.
Pajon R, Doria-Rose NA, Shen X, Schmidt SD, O'Dell S, McDanal C, Feng W, Tong J, Eaton A, Maglinao M, Tang H, Manning KE, Edara VV, Lai L, Ellis M, Moore KM, Floyd K, Foster SL, Posavad CM, Atmar RL, Lyke KE, Zhou T, Wang L, Zhang Y, Gaudinski MR, Black WP, Gordon I, Guech M, Ledgerwood JE, Misasi JN, Widge A, Sullivan NJ, Roberts PC, Beigel JH, Korber B, Baden LR, El Sahly H, Chalkias S, Zhou H, Feng J, Girard B, Das R, Aunins A, Edwards DK, Suthar MS, Mascola JR, Montefiori DC. SARS-CoV-2 Omicron Variant Neutralization after mRNA-1273 Booster Vaccination. N Engl J Med. 2022 Mar 17;386(11):1088-1091.
Widge AT, Rouphael NG, Jackson LA, Anderson EJ, Roberts PC, Makhene M, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, McDermott AB, Flach B, Lin BC, Doria-Rose NA, O'Dell S, Schmidt SD, Neuzil KM, Bennett H, Leav B, Makowski M, Albert J, Cross K, Edara VV, Floyd K, Suthar MS, Buchanan W, Luke CJ, Ledgerwood JE, Mascola JR, Graham BS, Beigel JH; mRNA-1273 Study Group. Durability of Responses after SARS-CoV-2 mRNA-1273 Vaccination. N Engl J Med. 2021 Jan 7;384(1):80-82.
Anderson EJ, Rouphael NG, Widge AT, Jackson LA, Roberts PC, Makhene M, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, McDermott AB, Flach B, Lin BC, Doria-Rose NA, O'Dell S, Schmidt SD, Corbett KS, Swanson PA 2nd, Padilla M, Neuzil KM, Bennett H, Leav B, Makowski M, Albert J, Cross K, Edara VV, Floyd K, Suthar MS, Martinez DR, Baric R, Buchanan W, Luke CJ, Phadke VK, Rostad CA, Ledgerwood JE, Graham BS, Beigel JH; mRNA-1273 Study Group. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N Engl J Med. 2020 Dec 17;383(25):2427-2438.
Gaudinski MR, Coates EE, Novik L, Widge A, Houser KV, Burch E, Holman LA, Gordon IJ, Chen GL, Carter C, Nason M, Sitar S, Yamshchikov G, Berkowitz N, Andrews C, Vazquez S, Laurencot C, Misasi J, Arnold F, Carlton K, Lawlor H, Gall J, Bailer RT, McDermott A, Capparelli E, Koup RA, Mascola JR, Graham BS, Sullivan NJ, Ledgerwood JE; VRC 608 Study team. Safety, tolerability, pharmacokinetics, and immunogenicity of the therapeutic monoclonal antibody mAb114 targeting Ebola virus glycoprotein (VRC 608): an open-label phase 1 study. Lancet. 2019 Mar 2;393(10174):889-898.
Training Programs
NIAID Allergy/Immunology Fellowship Program
- Phase 1, first-in-human clinical trials for novel vaccines and monoclonal antibodies for infectious diseases with a high public health burden as well as pandemic/biodefense threats and emerging infectious diseases
- Areas of expertise include SARS-CoV-2, influenza, Nipah, HIV, and Ebola
- Leads the VRC’s Pharmacovigilance Program
Alicia T. Widge, M.D.
Specialty(s): Allergy and Immunology, Pediatrics Provides direct clinical care to patients at NIH Clinical Center
Education:
B.A., 2006, Carleton College
M.S., 2011, Harvard School of Public Health
M.D., 2014, George Washington University School of Medicine and Health Sciences

Richard L. Wu, M.D.
Specialty(s): Allergy and Immunology, Internal Medicine Provides direct clinical care to patients at NIH Clinical Center
Education:
M.D., Uniformed Services University, Bethesda, MD
B.S., B.A., University of California, Berkeley

Richard L. Wu, M.D.
Richard Wu M.D. is a Principal Investigator and Medical Officer of multiple phase 1 clinical trials for vaccine candidates and monoclonal antibodies against infectious targets such as malaria, Ebola, COVID-19, HIV, influenza, and Nipah virus. He is responsible for designing, implementing, and providing oversight for clinical trials at the Vaccine Research Center. Additionally, he represents the Vaccine Research Center on collaborations with external academic and industry partners.
Park HJ, Brooks DI, Chavarria CS, Wu RL, Mikita CP, Beakes DE. Combining Discordant Serum IgE and Skin Testing Improves Diagnostic and Therapeutic Accuracy for Hymenoptera Venom Hypersensitivity Immunotherapy. J Allergy Clin Immunol Pract. 2022 Mar;10(3):837-843.e3.
Wu R, DiLorenzo A, Lotke M, Habeshian K, Brooks J, Keller MD, Kirkorian AY. Evaluation and Treatment of Febrile Ulceronecrotic Mucha-Habermann Disease With Ruxolitinib and Tocilizumab as Guided by Cytokine Profile. JAMA Dermatol. 2021 Nov 1;157(11):1381-1383.
Gaudinski MR, Berkowitz NM, Idris AH, Coates EE, Holman LA, Mendoza F, Gordon IJ, Plummer SH, Trofymenko O, Hu Z, Campos Chagas A, O'Connell S, Basappa M, Douek N, Narpala SR, Barry CR, Widge AT, Hicks R, Awan SF, Wu RL, Hickman S, Wycuff D, Stein JA, Case C, Evans BP, Carlton K, Gall JG, Vazquez S, Flach B, Chen GL, Francica JR, Flynn BJ, Kisalu NK, Capparelli EV, McDermott A, Mascola JR, Ledgerwood JE, Seder RA; VRC 612 Study Team. A Monoclonal Antibody for Malaria Preventionv. N Engl J Med. 2021 Aug 26;385(9):803-814.
Wu R, Lyons JJ. Hereditary Alpha-Tryptasemia: a Commonly Inherited Modifier of Anaphylaxis. Curr Allergy Asthma Rep. 2021 May 10;21(5):33.
- Vaccinology
- Allergy
- Immunology
- Infectious Diseases
Tracy J. Ruckwardt, Ph.D.
Education: Ph.D., 2003, University of Maryland, Baltimore

Tracy J. Ruckwardt, Ph.D.
The Respiratory Viruses Core (RVC) within the Molecular Immunoengineering Section (MIS) at the Vaccine Research Center is focused on the development and testing of countermeasures for respiratory diseases caused by RNA viruses. Activities in the RVC range from antigen design and preclinical immunogenicity testing to exploratory studies using samples from human clinical trials. We have a long-standing interest in respiratory syncytial virus (RSV), which can cause serious illness, particularly for infants and older adults, and have evaluated immune responses to the prefusion F subunit vaccine, DS-Cav1, in a phase I clinical trial. Clinical samples were assessed for neutralizing activity against RSV, and B cell and T cell responses to vaccination were also determined. We continue to explore adaptive immunity to RSV infection and vaccination in ongoing research projects.
Our work also includes a variety of resurging and emerging viral pathogens, including paramyxoviruses, Zika, SARS-CoV-2, and enterovirus D68 (EV-D68). This often involves assay and model development for proof-of-concept immunogenicity and protection studies. We perform a variety of serological assays and use tools such as multiparameter flow cytometry to evaluate cellular responses and for monoclonal antibody discovery. RVC efforts are closely integrated with that of the MIS, and often support VRC-wide vaccine development efforts.
Phung E, Chang LA, Mukhamedova M, Yang L, Nair D, Rush SA, Morabito KM, McLellan JS, Buchholz UJ, Mascola JR, Crank MC, Chen G, Graham BS, Ruckwardt TJ. Elicitation of pneumovirus-specific B cell responses by a prefusion-stabilized respiratory syncytial virus F subunit vaccine. Sci Transl Med. 2022 Jun 22;14(650):eabo5032.
Ruckwardt TJ, Morabito KM, Phung E, Crank MC, Costner PJ, Holman LA, Chang LA, Hickman SP, Berkowitz NM, Gordon IJ, Yamshchikov GV, Gaudinski MR, Lin B, Bailer R, Chen M, Ortega-Villa AM, Nguyen T, Kumar A, Schwartz RM, Kueltzo LA, Stein JA, Carlton K, Gall JG, Nason MC, Mascola JR, Chen G, Graham BS; VRC 317 study team. Safety, tolerability, and immunogenicity of the respiratory syncytial virus prefusion F subunit vaccine DS-Cav1: a phase 1, randomised, open-label, dose-escalation clinical trial. Lancet Respir Med. 2021 Oct;9(10):1111-1120.
DiPiazza AT, Leist SR, Abiona OM, Moliva JI, Werner A, Minai M, Nagata BM, Bock KW, Phung E, Schäfer A, Dinnon KH 3rd, Chang LA, Loomis RJ, Boyoglu-Barnum S, Alvarado GS, Sullivan NJ, Edwards DK, Morabito KM, Mascola JR, Carfi A, Corbett KS, Moore IN, Baric RS, Graham BS, Ruckwardt TJ. COVID-19 vaccine mRNA-1273 elicits a protective immune profile in mice that is not associated with vaccine-enhanced disease upon SARS-CoV-2 challenge. Immunity. 2021 Aug 10;54(8):1869-1882.e6.
DiPiazza AT, Graham BS, Ruckwardt TJ. T cell immunity to SARS-CoV-2 following natural infection and vaccination. Biochem Biophys Res Commun. 2021 Jan 29;538:211-217.
Ruckwardt TJ, Morabito KM, Graham BS. Immunological Lessons from Respiratory Syncytial Virus Vaccine Development. Immunity. 2019 Sep 17;51(3):429-442.
Crank MC, Ruckwardt TJ, Chen M, Morabito KM, Phung E, Costner PJ, Holman LA, Hickman SP, Berkowitz NM, Gordon IJ, Yamshchikov GV, Gaudinski MR, Kumar A, Chang LA, Moin SM, Hill JP, DiPiazza AT, Schwartz RM, Kueltzo L, Cooper JW, Chen P, Stein JA, Carlton K, Gall JG, Nason MC, Kwong PD, Chen GL, Mascola JR, McLellan JS, Ledgerwood JE, Graham BS; VRC 317 Study Team. A proof of concept for structure-based vaccine design targeting RSV in humans. Science. 2019 Aug 2;365(6452):505-509.
- Viral immunity and host/pathogen interactions
- Vaccine development for resurging and emerging
- infectious diseases, particularly respiratory RNA viruses
- Adaptive immune responses following vaccination or infection
Masaru Kanekiyo, D.V.M., Ph.D.
Education:
D.V.M., 2002, Nihon University
Ph.D., 2006, Nihon University

Masaru Kanekiyo, D.V.M., Ph.D.
Highlight
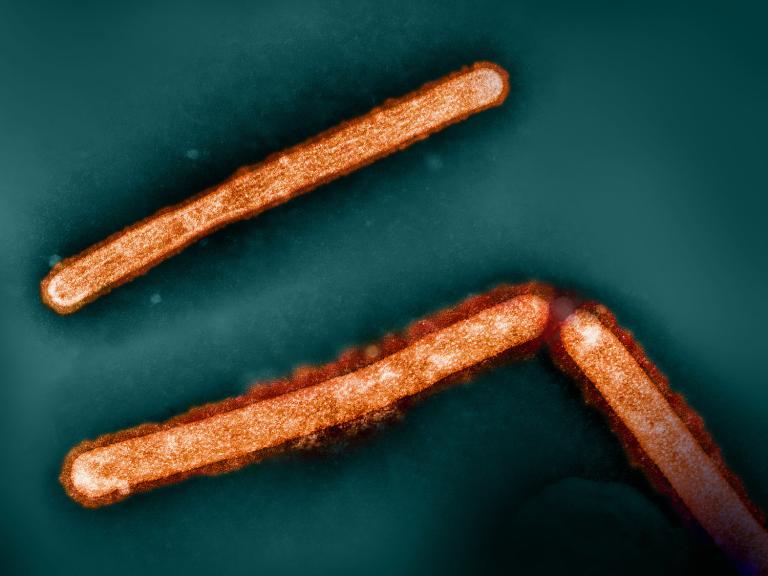
Single Dose of Broadly Neutralizing Antibody Protects Macaques from H5N1 Influenza
February 11, 2025
A single dose of a broadly neutralizing antibody given prior to virus exposure protects macaques from severe H5N1 avian influenza, NIH scientists report.
The Molecular Immunoengineering Section (MIS) at the Vaccine Research Center aims to conceive novel vaccine concepts that elicit broad and potent protective immune responses against influenza virus and provide a mechanistic principle for designing vaccines for other hypervariable pathogens such as coronaviruses and HIV-1. MIS is dedicated to advancing vaccine immunogen design beyond structure-based protein engineering by combining concepts and principles from multiple disciplines, including, but not limited to, immunobiology, biochemistry, biophysics, nanotechnology, and computational biology. The mission of the MIS is to define the fundamental rules behind vaccine-elicited immunity. Our interests span from basic immunology and virology to translational sciences with the goal of advancing vaccine concepts that would radically improve immune responses to vaccines. Our work involves various biochemical, biophysical, structural, immunological, and computational techniques and tools. MIS efforts include development of “supraseasonal” and “pre-pandemic” influenza vaccine candidates, “mosaic” antigen display technology, high-throughput high-definition virus neutralization assay systems, and animal models that recapitulate key aspects of human responses to influenza, such as immunological imprinting, preexisting immunity, antibody specificity and repertoire, immunodominance, and pathogenesis. MIS is also integrated with the VRC’s Influenza Program by serving as the lead of the Vaccine Concepts team. The Influenza Program involves multiple VRC Sections and Programs with the goal of advancing candidate vaccines from bench to clinic.
Ellis D, Lederhofer J, Acton OJ, Tsybovsky Y, Kephart S, Yap C, Gillespie RA, Creanga A, Olshefsky A, Stephens T, Pettie D, Murphy M, Sydeman C, Ahlrichs M, Chan S, Borst AJ, Park YJ, Lee KK, Graham BS, Veesler D, King NP, Kanekiyo M. Structure-based design of stabilized recombinant influenza neuraminidase tetramers. Nat Commun. 2022 Apr 5;13(1):1825.
Kanekiyo M, Graham BS. Next-Generation Influenza Vaccines. Cold Spring Harb Perspect Med. 2021 Aug 2;11(8):a038448.
Boyoglu-Barnum S, Ellis D, Gillespie RA, Hutchinson GB, Park YJ, Moin SM, Acton OJ, Ravichandran R, Murphy M, Pettie D, Matheson N, Carter L, Creanga A, Watson MJ, Kephart S, Ataca S, Vaile JR, Ueda G, Crank MC, Stewart L, Lee KK, Guttman M, Baker D, Mascola JR, Veesler D, Graham BS, King NP, Kanekiyo M. Quadrivalent influenza nanoparticle vaccines induce broad protection. Nature. 2021 Apr;592(7855):623-628.
Creanga A, Gillespie RA, Fisher BE, Andrews SF, Lederhofer J, Yap C, Hatch L, Stephens T, Tsybovsky Y, Crank MC, Ledgerwood JE, McDermott AB, Mascola JR, Graham BS, Kanekiyo M. A comprehensive influenza reporter virus panel for high-throughput deep profiling of neutralizing antibodies. Nat Commun. 2021 Mar 19;12(1):1722.
Kanekiyo M, Ellis D, King NP. New Vaccine Design and Delivery Technologies. J Infect Dis. 2019 Apr 8;219(Suppl_1):S88-S96.
Kanekiyo M, Joyce MG, Gillespie RA, Gallagher JR, Andrews SF, Yassine HM, Wheatley AK, Fisher BE, Ambrozak DR, Creanga A, Leung K, Yang ES, Boyoglu-Barnum S, Georgiev IS, Tsybovsky Y, Prabhakaran MS, Andersen H, Kong WP, Baxa U, Zephir KL, Ledgerwood JE, Koup RA, Kwong PD, Harris AK, McDermott AB, Mascola JR, Graham BS. Mosaic nanoparticle display of diverse influenza virus hemagglutinins elicits broad B cell responses. Nat Immunol. 2019 Mar;20(3):362-372.
- Vaccine design and immunoengineering
- Antigen display and delivery systems
- Immune profiling and antibody discovery
- Assay and animal model development
Joseph P. Casazza, M.D., Ph.D.
My work at the NIH concentrates on two aspects of HIV infection: the control of HIV-infection by the immunologic mechanism and the description of the changes in the CD4 T cell transcriptome caused by HIV-infection. In collaboration with individuals at the VRC, the California Institute of Technology and the Ragon Institute I am responsible for a clinical trial in which an AAV vector carries the coding sequence for VRC07, a potent broadly neutralizing Ab, into muscle cells of HIV-infected individuals on effective anti-retroviral therapy. In some individuals production of VRC07 occurred at ug/ml serum quantities for over 3 years. Although this level of VRC07 is not protective, this study shows that it is possible to side-step some of the difficulties in producing an immunogen capable of inducing a broadly neutralizing antibody by using a viral vector to transduction muscle cells. I have also established methods to identify and sort live HIV-infected CD4 T cells. Unlike matrix proteins, envelope proteins are fully mature when transported to the surface of CD4 T cells. By using fluorescently labeled broadly neutralizing antibodies that bind HIV envelope protein expressed on the surface of CD4 T cells, it is possible to use index sorting to identify live HIV-infected CD4 T cells. The transcriptomes of these cells are then characterized using RNA seq. We have used these methods to characterize the transcriptomes from HIV-infected peripheral CD4 T cells and in ACH2 cells transitioning from “latent-infection” to “active-infection”. These studies have allowed us to correlate markers of disease progression such as CD4 down regulation, viral RNA concentrations and viral RNA splice patterns, with activation of NF-B pathway and increased HIV-RNA transcription. We are currently using these methods to identify and characterize the longitudinal effect of SHIV infection on the CD4 T cell transcriptome of individual SHIV infected CD4 T cells from rhesus macaque lymph nodes.
VRC 200 (03-I-0263): Apheresis and Specimen Collection Procedures to Obtain Plasma, Peripheral Blood Mononuclear Cells (PBMCs) and Other Specimens for Research Studies- Associate Investigator.
VRC 323 (NIH 20-I-0145): A Phase I Open-Label Clinical Trial to Evaluate the Dose, Safety, Toerablity and Immunogenicity of an Influenza H10 Stabilized Stem Ferritin Vaccine, VRC-FLUNPF0103-VP, in Healthy Adults- Principal Investigator
VRC 325 (NIH000410): A Phase I Open-Label Clinical Trial to Evaluate the Dose, Safety, Tolerability and Immunogenicity of Mosaic Quadrivalent Influenza Vaccine Compared with a Licensed Inactiviated Seasona QIV, in Healthy Adults – Associate Investigator
VRC-603 (NIH-18-I-0030): A Phase 1 Dose-Escalation Study of the Safety of AAV8-VRC07 (VRC-HIVAAV070-00-GT) Recombinant AAV Vector Expressing VRC07 HIV-1 Neutralizing Antibody in Antiretroviral-Treated, HIV-1 Infected Adults with Controlled Viremia -Principal Investigator.
VRC609 (NIH 20-I-0096) A Phase I Open-Label Dose Escalation Study of the Safety and Pharmacokinetics of a Human Monoclonal Antibody, VRC-HIVMAB091-00-AB (N6LS), Administered Intravenously or Subcutaneously to Healthy Adults- Medical Officer
VRC611 (NIH 000536) A Phase I Safety and Pharmacokinetics Study to Evaluate a Human Monoclonal Antibody (mAb) VRC-HIVMAB0102-00-AB (CAP256V2LS) Administered Via Subcutaneous and Intravenous Injection in Healthy Adults- Medical Officer
VRC 614 (NIH 000536) A Phase 1, Dose Escalation, Open-Label Clinical Trial with Experimental Controlled Human Malaria Infections (CHMI) to Evaluate Safety and Protective Efficacy of an Anti-Malaria Human Monoclonal Antibody, VRC-MALMAB0114-00-AB (L9LS), in Healthy, Malaria-Naive Adult- Medical Officer
VRC 900 (10-I-0109): Evaluation of Tissue-Specific Immune Responses in Adults 18 Years of Age and Older -Associate Investigator.
Casazza JP, Cale EM, Narpala S, Yamshchikov GV, Coates EE, Hendel CS, Novik L, Widge AT, Apte P, Gordon I, Gaudinski MR, Conan-Cibotti M, Lin BC, Trofymenko O, Telscher S, Plummer SA, Wycuff D, Adams WC, Pandey JP, McDermott A, Roederer M, Sukienik AN, Doria-Rose NA, O’Dell S, Gall JG, Flach B, Nason MC, Saunders KO, Stein JA, Schwartz RM, Balazs AB, Baltimore D, Nabel GJ, Koup RA, Graham BS, Ledgerwood JE, Mascola JR and the VRC 603 Study Team (2022) Nat Med. 2022 May;28(5):1022-1030. doi: 10.1038/s41591-022-01762-x. Epub 2022 Apr 11.PMID: 35411076.
Pegu A, Xu L, DeMouth ME, Fabozzi G, March K, Almasri CG, Cully MD, Wang K, Yang ES, Dias J, Fennessey CM, Hataye J, Wei RR, Rao E, Casazza JP, Promsote W, Asokan M, McKee K, Schmidt SD, Chen X, Liu C, Shi W, Geng H, Foulds KE, Kao SF, Noe A, Li H, Shaw GM, Zhou T, Petrovas C, Todd JP, Keele BF, Lifson JD, Doria-Rose NA, Koup RA, Yang ZY, Nabel GJ, Mascola JR. Potent anti-viral activity of a trispecific HIV neutralizing antibody in SHIV-infected monkeys. Cell Rep. 2022 Jan 4;38(1):110199.
Hataye JM, Casazza JP, Best K, Liang CJ, Immonen TT, Ambrozak DR, Darko S, Henry AR, Laboune F, Maldarelli F, Douek DC, Hengartner NW, Yamamoto T, Keele BF, Perelson AS, Koup RA. Principles Governing Establishment versus Collapse of HIV-1 Cellular Spread. Cell Host Microbe. 2019 Dec 11;26(6):748-763.e20.
Casazza JP, Bowman KA, Adzaku S, Smith EC, Enama ME, Bailer RT, Price DA, Gostick E, Gordon IJ, Ambrozak DR, Nason MC, Roederer M, Andrews CA, Maldarelli FM, Wiegand A, Kearney MF, Persaud D, Ziemniak C, Gottardo R, Ledgerwood JE, Graham BS, Koup RA; VRC 101 Study Team. Therapeutic vaccination expands and improves the function of the HIV-specific memory T-cell repertoire. J Infect Dis. 2013 Jun 15;207(12):1829-40.
Casazza JP, Betts MR, Price DA, Precopio ML, Ruff LE, Brenchley JM, Hill BJ, Roederer M, Douek DC, Koup RA. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J Exp Med. 2006 Dec 25;203(13):2865-77.
- HIV Vaccines
- HIV pathogenesis


