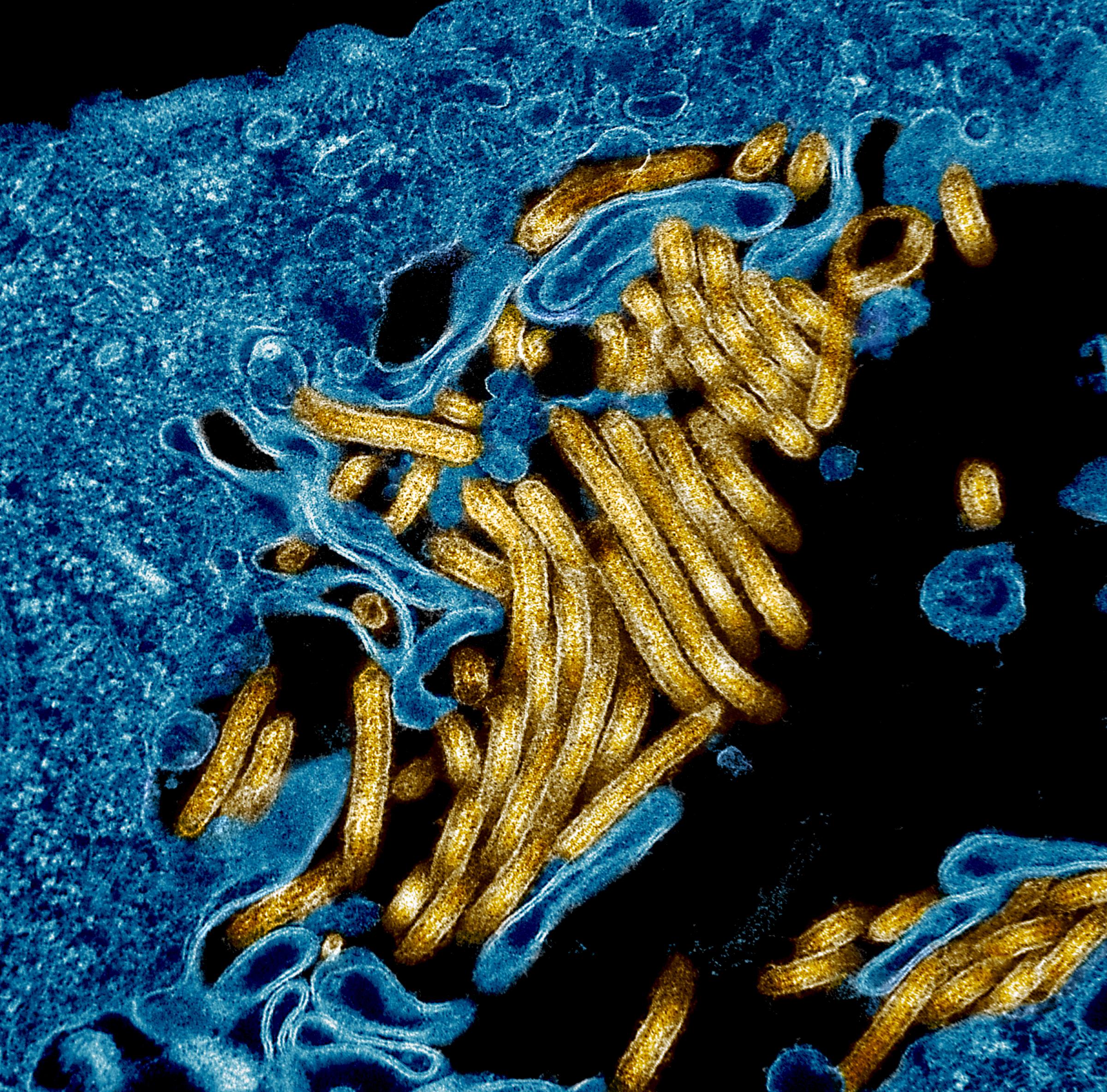
Currently there are no licensed vaccines to prevent Ebola virus disease. However, multiple investigational Ebola vaccines have been tested in numerous clinical trials around the world. NIAID has supported the development of various candidates, including the rVSV-ZEBOV vaccine developed by Merck. This candidate has been administered to people at risk of Ebola virus disease in the Democratic Republic of the Congo (DRC) and was previously tested in NIAID-supported clinical trials in West Africa.
rVSV-ZEBOV
The rVSV-ZEBOV vaccine uses a genetically engineered version of vesicular stomatitis virus (VSV), an animal virus that primarily affects cattle, to carry an Ebola virus gene insert. Experts at the Public Health Agency of Canada originally developed the vaccine, which is now licensed to Merck. NIAID and the Walter Reed Army Institute of Research (WRAIR) evaluated rVSV-ZEBOV in Phase 1 clinical trials which showed rVSV-ZEBOV is safe and able to induce a robust immune response in recipients.
NIAID, under a clinical research collaboration with the Liberian Ministry of Health known as PREVAIL, also conducted a Phase 2 randomized, placebo-controlled clinical trial of the vaccine in Liberia during the 2014-2016 outbreak of Ebola virus disease. The trial originally was designed to advance to Phase 3 and enroll 28,000 volunteers but was scaled back because the decline in new Ebola cases made it impossible to conduct the larger study. The trial ultimately enrolled 1500 participants and results indicated that the vaccine was well-tolerated and induced an immune response among participants.
Other partners in the research response to the Ebola outbreak in West Africa, including the U.S. Centers for Disease Control and Prevention (CDC), conducted additional studies of rVSV-ZEBOV. The WHO's trial involved vaccinating contacts of people with Ebola virus disease and contacts of those contacts on an immediate or delayed vaccination schedule.
NIAID also is part of an international consortium conducting an ongoing clinical trial of various vaccine regimens using rVSV-ZEBOV and another experimental prime-boost regimen known as Ad26.ZEBOV/MVA-BN-Filo. See PREVAIL 5 or PREVAC under Researching Ebola in Africa for more information.
cAd3-EBO
NIAID and Okairos (a company later acquired by GSK) developed an Ebola vaccine candidate (now licensed to the Sabin Vaccine Institute) that uses a chimpanzee adenovirus (cAd3) vector, or carrier, to deliver Ebola genetic material. The gene inserts express an Ebola virus protein designed to prompt the human body to make an immune response.
The cAd3-EBO Z vaccine (designed to protect against the Ebola Zaire virus species) proved to be safe and immunogenic when evaluated in numerous Phase 1 clinical trials, including at the NIH Clinical Center in Bethesda, Maryland. The vaccine candidate also was tested in multiple Phase 2 trials in both adults and children, including the Phase 2 PREVAIL 1 trial in Liberia. The PREVAIL findings indicated the vaccine was well-tolerated and induced an immune response in recipients.
The Sabin Vaccine Institute has also licensed the cAd3 platform to develop vaccines against Sudan virus, another Ebola virus species, and Marburg virus. NIAID and WRAIR are conducting a clinical trial examining the safety and dosage of an investigational vaccine designed to protect against Sudan virus (known as cAd3-EBO S) at Makerere University in Kampala, Uganda. NIAID and WRAIR also are conducting another Phase 1 trial of a vaccine designed to protect against Marburg virus (cAd3-Marburg) at the WRAIR Clinical Trials Center in Silver Spring, Maryland.
Ad26.ZEBOV and MVA-BN Filo
NIAID and other funding partners supported the development and preclinical and clinical testing of an investigational vaccine regimen designed to protect against the virus responsible for the 2014-2016 Ebola outbreak in West Africa and the ongoing outbreak in the DRC. The vaccine candidate combines the Ad26.ZEBOV vector (based on the AdVac platform developed by Crucell Holland B.V., a company acquired by Janssen Pharmaceutical Companies of Johnson & Johnson) with a modified vaccinia virus Ankara (MVA)-vectored vaccine (MVA-BN Filo, developed by Bavarian Nordic). NIAID, along with other groups, supported Phase 1 trials of the vaccine regimen. NIAID also is evaluating the prime-boost regimen in the PREVAIL 5 or PREVAC study in West Africa.
BARDA also is supporting advanced development of this candidate.
Additional Investigational Vaccines
NIAID is supporting research on additional recombinant vesicular stomatitis virus (VSV)-vectored vaccines against Ebola and Marburg viruses, a candidate nanoparticle Ebola vaccine, a parainfluenza type 3-vectored Ebola vaccine and a vaccine candidate based on an existing rabies vaccine that would protect against Ebola, Marburg, and rabies viruses