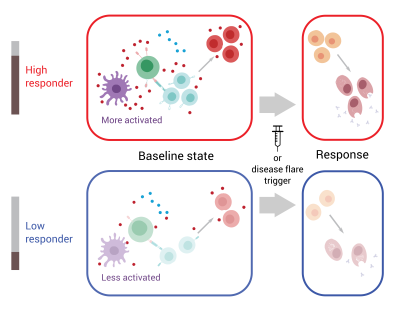
Model illustrating differences between people who are “high responders” and “low responders” according to the 10-gene signature. High responders have a more activated immune cell network at baseline—prior to vaccination for healthy people and during periods of remission for people with lupus—and are thus primed to more quickly mount an immune response to a stimulus.
Identifying predictors of autoimmune disease flare-ups could aid treatment for these conditions by allowing doctors to intervene before symptoms worsen. Recognizing immune system attributes that forecast a healthy person’s response to vaccination or infection would help researchers develop new vaccines.
A new NIAID study connects these seemingly distinct avenues of research. Using publicly available data, NIAID scientists identified features of a person’s immune state at “baseline”—prior to vaccination for healthy people and during periods of remission for people with the autoimmune disease systemic lupus erythematosus (lupus)—that predict later immune responses. Their findings, which appear February 24, 2020 in Nature Medicine, suggest that developing ways to alter an individual’s baseline immune state could improve responses to vaccines or reduce disease activity in people with autoimmune conditions.
Individual immune responses to vaccines vary, with some people producing more protective antibodies after vaccination than others. Scientists previously observed that a person’s immune profile prior to vaccination can offer clues about how their immune system will respond to the vaccine. Analogously, lupus symptoms vary in frequency and severity among individuals with the disease. Lupus involves periods of remission interspersed with symptom flare-ups, and researchers suspected that features of a person’s immune profile during remission may help predict flares.
Building off previous work, researchers from NIAID’s Laboratory of Immune System Biology and colleagues first analyzed data on gene expression—the degree to which genes are turned on or off—in blood samples taken from healthy individuals prior to seasonal influenza vaccination. They identified a pre-vaccination “signature” based on expression levels of 10 genes that distinguished people with a robust antibody response to the vaccine—the “high responders”—from those with a less robust, but still protective, antibody response—the so-called “low responders.”
The scientists next tested whether the same 10-gene signature could predict immune responses to the yellow fever vaccine. Unlike the seasonal flu vaccine, which contains killed influenza virus, the yellow fever vaccine contains a live version of the yellow fever virus that has been weakened so it cannot cause disease. The two vaccines stimulate the immune system by partially distinct mechanisms. Nevertheless, among people who had not previously been exposed to yellow fever, the 10-gene signature could accurately distinguish high and low vaccine responders.
The researchers suspected that the 10-gene signature also may be applicable to lupus. Immune responses to influenza and yellow fever are characterized by a rise in blood levels of plasmablasts, a specific type of immune cell. In some people with lupus, disease flares coincide with an increase in this cell type. Indeed, scientists found that measuring the 10-gene signature during remission could differentiate high and low levels of disease activity among people with lupus who experience plasmablast-associated flares.
Using a new experimental technique to simultaneously assess gene and protein expression in individual cells, the scientists next aimed to home in on the cells involved in establishing the immune system’s baseline state. They found that an entire network of different cell types is involved. Among high responders, this immune cell network is in an activated state, while it is less activated among low responders. The high responders are thus primed to more quickly mount an immune response to a stimulus. Senior author John Tsang, Ph.D., compares it to preparing for a road race. If all the participants are lined up at the start line and ready to go, the race begins quickly when the gun goes off. But if the runners are scattered and unprepared before the start, it takes much longer for the race to get underway.
Next, the NIAID team hopes to unravel the factors that lead some people to have a more activated baseline immune state. In other words, what makes a person a high responder? The 10-gene signature identified in the current study did not reflect a person’s age, and the signature predicted high and low responders among men or women. However, women tended to have higher expression levels of the 10 genes than men, consistent with previous observations that women generally mount stronger immune responses to vaccination and are more prone to autoimmune diseases. Ultimately, clinical trials will be necessary to evaluate the applicability of such immune signatures across different populations and diseases.
The NIAID scientists now are searching for similar baseline immune response determinants in different populations and also assessing genetics, environmental exposures, and other factors that may play roles in determining the immune system’s baseline state. They have made all the data used in their study, as well as computer source codes packaged in software containers for reproducibility, freely available online for others to use or adapt to answer their own research questions.
Reference:
Y Kotliarov, R Sparks et al. Broad immune activation underlies shared set point signatures for vaccine responsiveness in healthy individuals and disease activity in patients with lupus. Nature Medicine DOI: 10.1038/s41591-020-0769-8 (2020).
The datasets used for analysis in this study were generated by various sources, including the NIAID-funded Human Immunology Project Consortium and the NIH Center for Human Immunology. These data are also available from the NIAID-supported ImmPort database.