Respiratory syncytial virus (RSV) is a common respiratory virus that usually causes mild, cold-like symptoms. However, RSV can cause serious illness or death in premature or very young infants and people over age 65, highlighting a critical need for vaccines in these populations.
Journey to a Better Vaccine
After NIH scientists identified RSV as a human pathogen in 1957, researchers tested multiple vaccines that proved unsuccessful, leading scientists to explore RSV surface proteins as a vaccine target for pregnant people (to protect the newborn) and the elderly.
-
1980s
Scientists identified the RSV fusion “F” protein, a surface protein that helps the virus infect human cells.
-
1990s
NIAID researchers found mice exposed to the RSV F protein produced a more protective immune response compared to mice exposed to other surface proteins.
-
2000s
Two forms of the RSV F protein were identified, later determined to be the prefusion and postfusion states.
-
2010s
NIAID scientists locked the RSV F protein in its prefusion state, which was a more protective vaccine candidate. Read more below.
-
2020s
Vaccine candidates using the locked RSV prefusion F protein began Phase 3 clinical trials.
Two States of the RSV F Protein
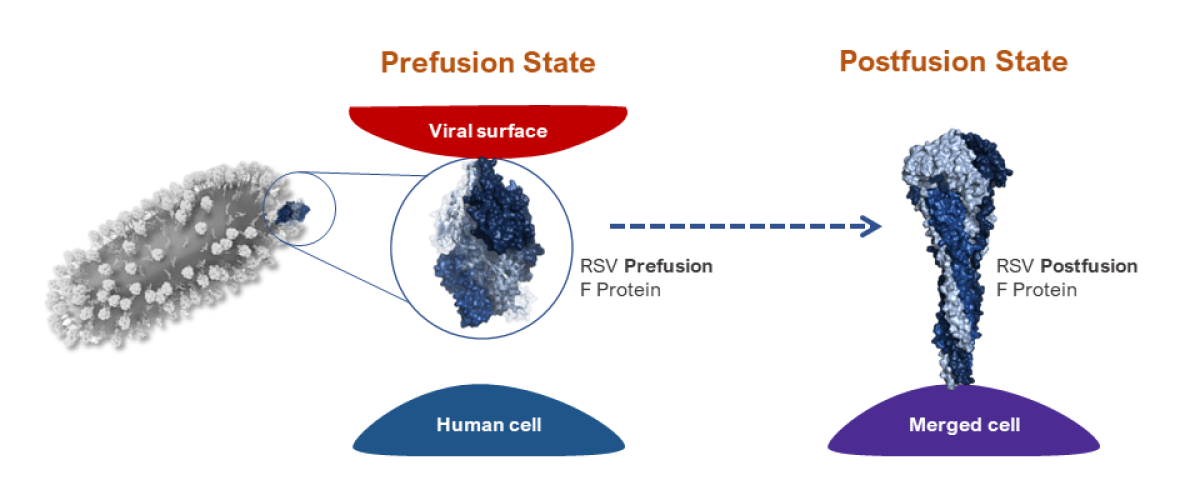
Prefusion State: In its prefusion state, the RSV F protein is attached to the viral surface and helps the virus attach to host cells.
Postfusion State: After the virus recognizes a human cell, the F protein irreversibly transitions from its prefusion to its postfusion state, and the virus merges with the human cell. This allows the virus to infect the host.
Preclinical Findings Show Promise for Prefusion F Protein Vaccines
NIAID found animals vaccinated with prefusion F protein developed a more potent immunity than those vaccinated with the postfusion F protein. This discovery led scientists toward finding a way to prevent the F protein from transitioning from its prefusion state to its postfusion state.
NIAID Scientific Breakthrough: Stabilized Prefusion F Protein
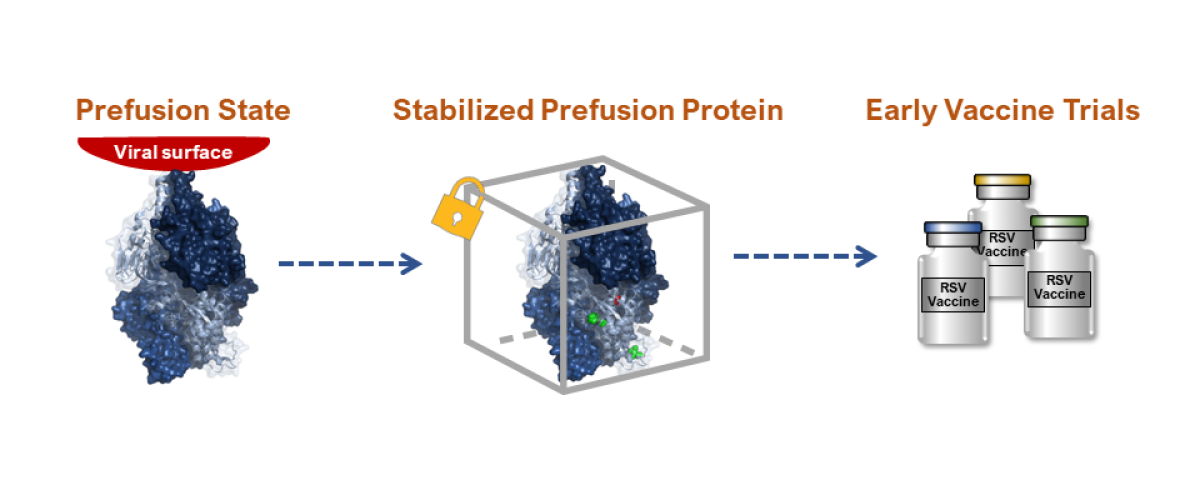
Prefusion State: When the F protein is attached to the virus, it remains in a prefusion conformation, which was shown in animals to be more protective.
Locked Prefusion F Protein: NIAID scientists identified a way to lock the RSV F protein in its prefusion state, preventing it from transitioning to the postfusion state.
Early Vaccine Trials: NIAID’s early clinical trials with a locked prefusion F protein vaccine proved safe and induced an immune response, leading the private sector to develop similar experimental vaccines.
Download a printable version of the RSV protein vaccine story


