Novel Study Model Reveals New Understanding of Fatal Familial Insomnia
Cerebral Organoids Show NIAID Investigators Disease Characteristics
Fatal familial insomnia (FFI) is a little-known yet horrific disease in which people die from lack of sleep. A protein mutation in the brain prevents sleep, and the body gradually deteriorates. Fortunately, the disease is extremely rare. Fewer than 1,000 people in the United States are estimated to have FFI, according to the NIH’s Genetic and Rare Diseases Information Center. Unfortunately for those with the disease, it can be hereditary – thus the “familial” aspect in the name and importance of developing diagnostic tests and treatments.
FFI is among a group of unusual neurologic conditions known as prion diseases – those caused by normally harmless prion protein that can malfunction and kill brain cells. Because FFI is found in the brain (suspected origin is the thalamus) studying its spread is not possible until a patient has died. But at that point, valuable disease information is not available because the brain no longer functions. Likewise, studies in rodents and laboratory glassware only have provided limited information.
In a new study published in PLOS Genetics, scientists from the National Institute of Allergy and Infectious Diseases (NIAID) developed a cerebral organoid model to study the exact protein mutation that causes FFI. Human cerebral organoids are small balls of brain cells ranging in size from a poppy seed to a pea; scientists use human skin cells to create organoids. Cerebral organoids have organization, structure, and electrical signaling systems similar to human brain tissue. Because they can survive in a controlled environment for months to years, cerebral organoids also are ideal for studying nervous system diseases over lengthy periods of time.
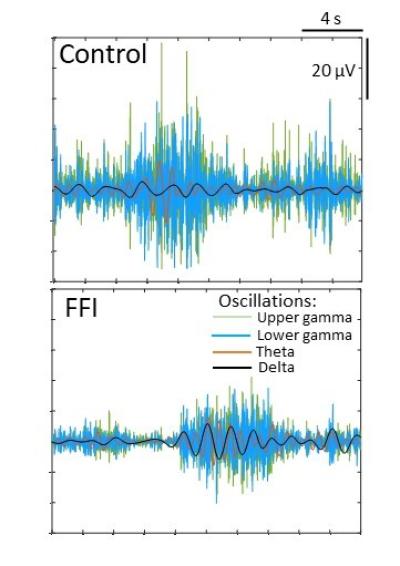
In the new study using the FFI organoids, NIAID scientists working at Rocky Mountain Laboratories in Hamilton, Mont., compared cell functions – primarily in neurons – between the FFI model and organoids without the FFI protein mutation, making several important observations about the mutation’s effect on brain cells.
They believe the abnormalities likely are features of asymptomatic FFI that may lead to disease.
They surprisingly did not observe spontaneous change in shape or spread of the FFI protein to additional prion protein. Such spread typically is a trademark of prion disease – changing prion protein throughout the brain from the normal shape to the malfunctioning folded shape.
“Our findings show that the mutation causes brain cells to dysfunction without the need for misfolding,” the study states. “We could confirm that most changes were caused by the presence of the mutation” rather than interacting with prion protein lacking the mutation.
Further, the group found that neurons in the FFI organoid model were impaired because of damaged mitochondria – which typically produce energy to keep the brain cells healthy. In future studies they hope to establish a relationship between impaired mitochondria function and the mutated FFI prion protein, and whether neurons attempt to stay healthy and avoid harm from the mutated FFI prion protein by switching from mitochondria as an energy source.
They also plan to explore relationships between the mutated FFI prion protein and neurons associated with “wakefulness,” sleep and rapid eye movement in the brain.
Reference:
S Foliaki et al. Altered energy metabolism in Fatal Familial Insomnia cerebral organoids is associated with astrogliosis and neuronal dysfunction. PLOS Genetics DOI: (2023).