134 Results
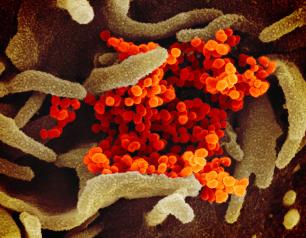
NIH Launches Clinical Trial of Epstein-Barr Virus Vaccine
May 6, 2022
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, has launched an early-stage clinical trial to evaluate an investigational preventative vaccine for Epstein-Barr virus (EBV). EBV is the primary cause of infectious mononucleosis and is associated with certain cancers and autoimmune diseases. The Phase 1 study, which will be conducted at the NIH Clinical Center in Bethesda, Maryland, is one of only two studies to test an investigational EBV vaccine in more than a decade.
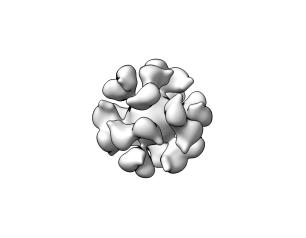
NIAID Marks World Malaria Day
April 25, 2022
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), reaffirms our commitment to developing safe and effective medical tools against malaria, and, in the words of this year’s World Malaria Day theme, “Harnessing innovation to reducing the malaria disease burden and saving lives.”
NIH Funds New Tuberculosis Research Advancement Centers
April 6, 2022
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, today announced four new grant awards to establish Tuberculosis Research Advancement Centers (TRACs)
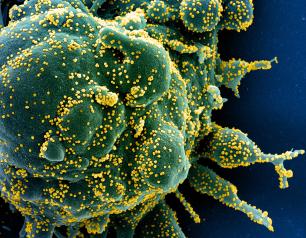
NIH Begins Clinical Trial Evaluating Second COVID-19 Booster Shots in Adults
March 31, 2022
A Phase 2 clinical trial evaluating various additional COVID-19 booster shots has begun enrolling adult participants in the United States. The trial aims to understand if different vaccine regimens—prototype and variant vaccines alone and in combinations—can broaden immune responses in adults who already have received a primary vaccination series and a first booster shot. The study, known as the COVID-19 Variant Immunologic Landscape (COVAIL) trial, is sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health.

NIH Experts Discuss Controlling COVID-19 in Commentary on Herd Immunity
March 31, 2022
Achieving classical herd immunity against SARS-CoV-2, the virus that causes COVID-19, may not be attainable, according to a new perspective published in The Journal of Infectious Diseases. However, widespread use of currently available public health interventions to prevent and control COVID-19 will enable resumption of most activities of daily life with minimal disruption.
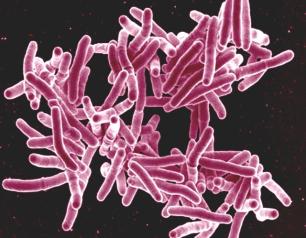
World TB Day 2022—Invest to End TB. Save Lives
March 24, 2022
Today marks the 140th anniversary of the announcement by Dr. Robert Koch that tuberculosis (TB) is caused by the bacterium Mycobacterium tuberculosis. World TB Day is a reminder that this ancient disease remains a relentless killer. The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, affirms its commitment to the 2022 World TB Day theme, Invest to End TB. Save Lives, by supporting and conducting wide-ranging research aimed at reducing the health and economic impacts of TB.
NIH Launches Clinical Trial of Three mRNA HIV Vaccines
March 14, 2022
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, has launched a Phase 1 clinical trial evaluating three experimental HIV vaccines based on a messenger RNA (mRNA) platform—a technology used in several approved COVID-19 vaccines. NIAID is sponsoring the study, called HVTN 302, and the NIAID-funded HIV Vaccine Trials Network (HVTN), based at Fred Hutchinson Cancer Research Center in Seattle, is conducting the trial.
NIH Launches Trial to Study Allergic Reactions to COVID-19 mRNA Vaccine
March 9, 2022
Researchers from the National Institute of Allergy and Infectious Diseases (NIAID) are conducting a clinical trial designed to help understand rare but potentially serious systemic allergic reactions to COVID-19 mRNA vaccines.
Leadership Transition at the NIAID Vaccine Research Center
February 16, 2022
Dr. Fauci expresses gratitude to John R. Mascola, M.D., as he announces his retirement as Director of the Dale and Betty Bumpers Vaccine Research Center at the National Institute of Allergy and Infectious Diseases.
Researchers Document Third Known Case of HIV Remission Involving Stem Cell Transplant
February 15, 2022
A woman with HIV who received a cord blood stem cell transplant to treat acute myeloid leukemia has had no detectable levels of HIV for 14 months despite cessation of antiretroviral therapy (ART), according to a presentation at today’s Conference on Retroviruses and Opportunistic Infections (CROI).
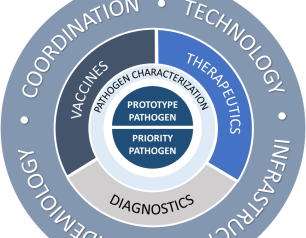
NIAID Pandemic Preparedness Plan Targets ‘Prototype’ and Priority Pathogens
February 2, 2022
The National Institute of Allergy and Infectious Diseases is focusing on preparing for a range of other viral threats that could cause a public health emergency, and according to NIAID’s new Pandemic Preparedness Plan, the institute will direct its preparedness efforts on two fronts.
NIH Supports Valley Fever Research with New Awards
February 2, 2022
The National Institute of Allergy and Infectious Diseases has announced three awards to establish a network of Coccidioidomycosis Collaborative Research Centers to conduct research on coccidioidomycosis, also known as Valley Fever.
Trial Tests Strategy to Augment Response to COVID-19 Vaccines in Transplant Recipients
January 31, 2022
A study has begun to assess the antibody response to an additional dose of a COVID-19 mRNA vaccine in kidney and liver transplant recipients, either alone or with a concurrent reduction in immunosuppressive medication.
Hyperimmune Intravenous Immunoglobulin Does Not Improve Outcomes for Adults Hospitalized with COVID-19
January 27, 2022
A clinical trial has found that the combination of remdesivir plus a highly concentrated solution of antibodies that neutralize SARS-CoV-2, the virus that causes COVID-19, is not more effective than remdesivir alone for treating adults hospitalized with the disease.
Mix-and-Match Trial Finds Additional Dose of COVID-19 Vaccine Safe, Immunogenic
January 26, 2022
In adults who had previously received a full regimen of any of three COVID-19 vaccines granted Emergency Use Authorization (EUA) or approved by the Food and Drug Administration (FDA), an additional booster dose of any of these vaccines was safe and prompted an immune response, according to preliminary clinical trial results reported in The New England Journal of Medicine. The findings served as the basis for recommendations by the FDA and the Centers for Disease Control and Prevention in late fall 2021 to permit mix-and-match COVID-19 booster vaccinations in the United States.
Statement—NIH Celebrates FDA Approval of Long-Acting Injectable Drug for HIV Prevention
December 21, 2021
U.S. Food and Drug Administration announced its first approval of a long-acting HIV prevention medication. Developed by ViiV Healthcare, the medicine is long-acting cabotegravir injected once every two months. FDA has approved the medicine for use by adults and adolescents weighing at least 35 kilograms who are at risk of sexually acquiring HIV.
Experimental mRNA HIV Vaccine Safe, Shows Promise in Animals
December 9, 2021
An experimental HIV vaccine based on mRNA—the same platform technology used in two highly effective COVID-19 vaccines—shows promise in mice and non-human primates, according to scientists at the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health.
NIH Statement on World AIDS Day
December 1, 2021
Since 1988, World AIDS Day has been an annual call to end the HIV/AIDS pandemic as we remember the many who lost their lives to the disease. Considerable progress has been made since the first World AIDS Day; however, far too many people continue to acquire HIV and die from its related illnesses.
Too Many People with HIV Fail to Achieve Durable Viral Suppression
November 29, 2021
Among people with HIV worldwide who are receiving antiretroviral therapy (ART), adults are getting closer to the global target of 95% achieving viral suppression, but progress among children and adolescents is lagging and long-term viral suppression among all groups remains a challenge.
Lung Autopsies of COVID-19 Patients Reveal Treatment Clues
November 17, 2021
Lung autopsy and plasma samples from people who died of COVID-19 have provided a clearer picture of how the SARS-CoV-2 virus spreads and damages lung tissue.
NIH Researchers Identify How Two People Controlled HIV After Stopping Treatment
October 28, 2021
Research led by scientists at the National Institutes of Health has identified two distinct ways that people with HIV can control the virus for an extended period after stopping antiretroviral therapy (ART) under medical supervision. This information could inform efforts to develop new tools to help people with HIV put the virus into remission without taking lifelong medication, which can have long-term side-effects.
Subcutaneous Interferon Does Not Improve Outcomes for Hospitalized Adults with COVID-19
October 18, 2021
A clinical trial has found that treatment with the immunomodulator interferon beta-1a plus the antiviral remdesivir was not superior to treatment with remdesivir alone in hospitalized adults with COVID-19 pneumonia. In addition, in a subgroup of patients who required high-flow oxygen, investigators found that interferon beta-1a was associated with more adverse events and worse outcomes. These findings were published today in the journal The Lancet Respiratory Medicine.
NIAID Issues New Awards to Fund “Pan-Coronavirus” Vaccines
September 28, 2021
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, has awarded approximately $36.3 million to three academic institutions to conduct research to develop vaccines to protect against multiple types of coronaviruses and viral variants. The awards are intended to fuel vaccine research for a diverse family of coronaviruses, with a primary focus on potential pandemic-causing coronaviruses, such as SARS-CoV-2.
Investigational Malaria Vaccine Gives Strong, Lasting Protection
June 30, 2021
Two U.S. Phase 1 clinical trials of a novel candidate malaria vaccine have found that the regimen conferred unprecedentedly high levels of durable protection when volunteers were later exposed to disease-causing malaria parasites. The vaccine combines live parasites with either of two widely used antimalarial drugs—an approach termed chemoprophylaxis vaccination. A Phase 2 clinical trial of the vaccine is now underway in Mali, a malaria-endemic country.
Adjuvant Developed with NIH Funding Enhances Efficacy of India’s COVID-19 Vaccine
June 29, 2021
An adjuvant developed with funding from the National Institutes of Health has contributed to the success of the highly efficacious COVAXIN COVID-19 vaccine, which roughly 25 million people have received to date in India and elsewhere. Adjuvants are substances formulated as part of a vaccine to boost immune responses and enhance a vaccine’s effectiveness. COVAXIN was developed and is manufactured in India, which is currently suffering a devastating health crisis due to COVID-19.