40 Results
NIH-Sponsored Trial of Lassa Vaccine Opens
March 17, 2025
An NIAID-sponsored clinical trial of an experimental vaccine to prevent Lassa fever has begun.
NIH-Funded Clinical Trial Will Evaluate New Dengue Therapeutic
February 11, 2025
A Phase 2 clinical trial will test the safety and efficacy of an experimental treatment for dengue, a viral disease transmitted by mosquitoes.
Single Dose of Broadly Neutralizing Antibody Protects Macaques from H5N1 Influenza
February 11, 2025
A single dose of a broadly neutralizing antibody given prior to virus exposure protects macaques from severe H5N1 avian influenza, NIH scientists report.
As Prevention Strategy for Sexually Transmitted Infections Rolls Out, Experts Highlight both Promise and Knowledge Gaps
January 6, 2025
DoxyPEP is reducing the rate of syphilis and chlamydia but has had little to no effect on gonorrhea and needs close monitoring for antibiotic resistance.
NIH Officials Assess Threat of H5N1
December 31, 2024
HPAI H5N1 influenza remains a low risk to most Americans, but that does not diminish concern about the virus, NIAID experts say.
Single Mutation in H5N1 Influenza Surface Protein Could Enable Easier Human Infection
December 6, 2024
A single modification in the protein found on the surface of the highly pathogenic avian influenza (HPAI) H5N1 influenza virus currently circulating in U.S. dairy cows could allow for easier transmission among humans, according to new research funded by the National Institutes of Health (NIH) and published today in the journal Science. The study results reinforce the need for continued, vigilant surveillance and monitoring of HPAI H5N1 for potential genetic changes that could make the virus more transmissible in humans.
Bovine H5N1 Influenza from Infected Worker Transmissible and Lethal in Animal Models
October 28, 2024
Bovine H5N1 influenza virus taken from eye of infected worker transmissible and lethal in animal models.
Mpox Vaccine Is Safe and Generates a Robust Antibody Response in Adolescents
October 16, 2024
A clinical trial of an mpox vaccine in adolescents found it was safe and generated an antibody response equivalent to that seen in adults. Results were presented at IDWeek2024.
NIH Awards Establish Pandemic Preparedness Research Network
September 13, 2024
The Research and Development of Vaccines and Monoclonal Antibodies for Pandemic Preparedness network—called ReVAMPP—will focus its research efforts on “prototype pathogens,” representative pathogens from virus families known to infect humans, and high-priority pathogens that have the potential to cause deadly diseases. The pandemic preparedness research network will conduct research on high-priority pathogens most likely to threaten human health with the goal of developing effective vaccines and monoclonal antibodies.

Emergency Department Screening More Than Doubles Detection of Syphilis Cases
September 10, 2024
Providing optional syphilis tests to most people seeking care at a large emergency department led to a dramatic increase in syphilis screening and diagnosis, according to study of nearly 300,000 emergency department encounters in Chicago. Most people diagnosed had no symptoms, which suggests that symptom-based testing strategies alone could miss opportunities to diagnose and treat people with syphilis.
NIH Awards Will Support Innovation in Syphilis Diagnostics
September 3, 2024
NIAID has awarded grants for 10 projects to improve diagnostic tools for congenital and adult syphilis—conditions currently diagnosed with a sequence of tests, each with limited precision. The Centers for Disease Control and Prevention estimates that adult and congenital syphilis cases increased by 80% and 183% respectively between 2018 and 2022—a crisis that prompted the U.S. Department of Health and Human Services (HHS) to establish a national taskforce to respond to the epidemic.
Features of H5N1 Influenza Viruses in Dairy Cows May Facilitate Infection, Transmission in Mammals
July 8, 2024
A series of experiments with highly pathogenic H5N1 avian influenza (HPAI H5N1) viruses circulating in infected U.S. dairy cattle found that viruses derived from lactating dairy cattle induced severe disease in mice and ferrets when administered via intranasal inoculation.
NIH-Sponsored Trial of Nasal COVID-19 Vaccine Opens
July 1, 2024
A Phase 1 trial testing the safety of an experimental nasal vaccine that may provide enhanced breadth of protection against emerging variants of SARS-CoV-2, the virus that causes COVID-19, is now enrolling healthy adults at three sites in the United States.
NIH-Sponsored Trial of Enterovirus D68 Therapeutic Begins
June 27, 2024
The National Institutes of Health (NIH) is sponsoring a clinical trial to evaluate the safety of an investigational monoclonal antibody to treat enterovirus D68 (EV-D68), which can cause severe respiratory and neurological diseases such as acute flaccid myelitis (AFM) – similar to polio. Scientists are striving to better understand AFM, which has emerged in the United States with spikes in cases every other year, primarily in the late-summer months over the last decade. The U.S. Centers for Disease Control and Prevention (CDC) identified increases in AFM cases in 2014, 2016, and 2018. EV-D68 is a virus of growing public health concern due to its association with the intermittent AFM outbreaks.
High H5N1 Influenza Levels Found in Mice Given Raw Milk from Infected Dairy Cows
May 24, 2024
Mice administered raw milk samples from dairy cows infected with H5N1 influenza experienced high virus levels in their respiratory organs and lower virus levels in other vital organs, according to findings published in the New England Journal of Medicine. The results suggest that consumption of raw milk by animals poses a risk for H5N1 infection and raises questions about its potential risk in humans.
Lower Dose of Mpox Vaccine Is Safe and Generates Six-Week Antibody Response Equivalent to Standard Regimen
April 27, 2024
A dose-sparing intradermal mpox vaccination regimen was safe and generated an antibody response equivalent to that induced by the standard regimen at six weeks (two weeks after the second dose), according to findings presented today at the European Society of Clinical Microbiology and Infectious Diseases Global Congress in Barcelona. The results suggest that antibody responses contributed to the effectiveness of dose-sparing mpox vaccine regimens used during the 2022 U.S. outbreak.
World TB Day 2024 – Yes! We Can End TB!
March 22, 2024
In observance of World Tuberculosis Day (Sunday, March 24), NIAID joins our partners in reaffirming our commitment to ending the tuberculosis (TB) pandemic while honoring the lives lost to TB disease.
COVID-19 Vaccination and Boosting During Pregnancy Protects Infants for Six Months
February 14, 2024
Women who receive an mRNA-based COVID-19 vaccination or booster during pregnancy can provide their infants with strong protection against symptomatic COVID-19 infection for at least six months after birth. These findings reinforce the importance of receiving both a COVID-19 vaccine and booster during pregnancy to ensure that infants are born with robust protection that lasts until they are old enough to be vaccinated.

NIH Statement on Preliminary Efficacy Results of First-in-Class Gonorrhea Antibiotic Developed Through Public-Private Partnership
November 1, 2023
A single dose of a novel oral antibiotic called zoliflodacin has been found to be as safe and effective as standard therapy for uncomplicated urogenital gonorrhea in an international Phase 3 non-inferiority clinical trial. Gonorrhea treatment options are increasingly limited due to antimicrobial resistance seen in Neisseria gonorrhoeae, the bacteria that cause gonococcal infection.

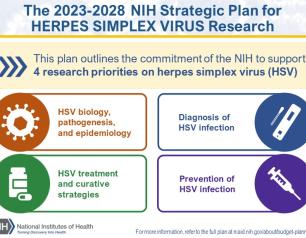
NIH Releases Strategic Plan for Research on Herpes Simplex Virus 1 and 2
September 19, 2023
In response to the persistent health challenges of herpes simplex virus 1 (HSV-1) and HSV-2, an NIH-wide HSV Working Group developed the plan, informed by feedback from more than 100 representatives of the research and advocacy communities and interested public stakeholders. The plan outlines an HSV research framework with four strategic priorities: improving fundamental knowledge of HSV biology, pathogenesis, and epidemiology; accelerating research to improve HSV diagnosis; improving strategies to treat HSV while seeking a curative therapeutic; and, advancing research to prevent HSV infection.
COVID-19 Vaccination and Boosting During Pregnancy Benefits Pregnant People and Newborns
August 11, 2023
Receiving a COVID-19 mRNA vaccine or booster during pregnancy can benefit pregnant people and their newborn infants, according to findings recently published in Vaccine. The paper describes results from the Multisite Observational Maternal and Infant Study for COVID-19 (MOMI-VAX), which was funded by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health.
NIH Awards Will Fund Post-Treatment Lyme Disease Syndrome Research
July 21, 2023
Five projects awarded for research to better understand Post-treatment Lyme Disease Syndrome (PTLDS), which is a collection of symptoms, such as pain, fatigue, and difficulty thinking or “brain fog,” that linger following standard treatment for Lyme disease.

Clinical Trial of mRNA Universal Influenza Vaccine Candidate Begins
May 15, 2023
A clinical trial of an experimental universal influenza vaccine developed by researchers at the National Institute of Allergy and Infectious Diseases’ (NIAID) Vaccine Research Center (VRC), part of the National Institutes of Health, has begun enrolling volunteers at Duke University in Durham, North Carolina. This Phase 1 trial will test the experimental vaccine, known as H1ssF-3928 mRNA-LNP, for safety and its ability to induce an immune response.
NIAID Marks World Malaria Day
April 25, 2023
World Malaria Day is an opportunity to reflect on continuing challenges posed by malaria and reaffirm a commitment to overcoming them. The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, joins with the global health community in recognizing this year’s theme of “Time to Deliver on Zero Malaria: Invest, Innovate, Implement.”
The Potential and Challenges of Mucosal COVID-19 Vaccines
April 13, 2023
In November 2022, the National Institute of Allergy and Infectious Diseases (NIAID) co-hosted a virtual workshop on the importance and challenges of developing mucosal vaccines for SARS-COV-2. The highlights of this workshop have now been published as a report in npj Vaccines.