152 Results
NIH-Sponsored Trial of Nasal COVID-19 Vaccine Opens
July 1, 2024
A Phase 1 trial testing the safety of an experimental nasal vaccine that may provide enhanced breadth of protection against emerging variants of SARS-CoV-2, the virus that causes COVID-19, is now enrolling healthy adults at three sites in the United States. The National Institutes of Health (NIH) is sponsoring the first-in-human trial of the investigational vaccine, which was designed and tested in pre-clinical studies by scientists from NIH’s National Institute of Allergy and Infectious Diseases (NIAID) Laboratory of Infectious Diseases.
NIH-Sponsored Trial of Enterovirus D68 Therapeutic Begins
June 27, 2024
The National Institutes of Health (NIH) is sponsoring a clinical trial to evaluate the safety of an investigational monoclonal antibody to treat enterovirus D68 (EV-D68), which can cause severe respiratory and neurological diseases such as acute flaccid myelitis (AFM) – similar to polio. Scientists are striving to better understand AFM, which has emerged in the United States with spikes in cases every other year, primarily in the late-summer months over the last decade. The U.S. Centers for Disease Control and Prevention (CDC) identified increases in AFM cases in 2014, 2016, and 2018. EV-D68 is a virus of growing public health concern due to its association with the intermittent AFM outbreaks.
NIH Statement on Preliminary Efficacy Results of Twice-Yearly Lenacapavir for HIV Prevention in Cisgender Women
June 26, 2024
The injectable antiretroviral drug lenacapavir was safe and 100% effective as long-acting HIV pre-exposure prophylaxis (PrEP) among cisgender women in a Phase 3 clinical trial, according to top-line findings released by Gilead Sciences, Inc., the study sponsor. Lenacapavir is administered every six months, making it the most durable HIV prevention method to have shown efficacy in this population.
Infectious H5N1 Influenza Virus in Raw Milk Rapidly Declines with Heat Treatment
June 14, 2024
The amount of infectious H5N1 influenza viruses in raw milk rapidly declined with heat treatment in laboratory research conducted by scientists at the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health. However, small, detectable amounts of infectious virus remained in raw milk samples with high virus levels when treated at 72 degrees Celsius (161.6 degrees Fahrenheit) for 15 seconds—one of the standard pasteurization methods used by the dairy industry.

NIH Releases H5N1 Influenza Research Agenda
June 5, 2024
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, has released its plan for advancing H5N1 influenza basic research and translating those findings into strategies and interventions that can benefit people.
U.S. Clinical Trials Begin for Twice-Yearly HIV Prevention Injection
June 4, 2024
Two clinical trials have launched to examine a novel long-acting form of HIV pre-exposure prophylaxis (PrEP) in cisgender women and people who inject drugs. The mid-stage studies will assess the safety, acceptability, and pharmacokinetics (how a drug moves through the body) of lenacapavir, an antiretroviral drug administered by injection every six months. The studies are sponsored and funded by Gilead Sciences, Inc., and implemented through the HIV Prevention Trails Network (HPTN).
Novel Vaccine Concept Generates Immune Responses that Could Produce Multiple Types of HIV Broadly Neutralizing Antibodies
May 30, 2024
Using a combination of cutting-edge immunologic technologies, researchers have successfully stimulated animals’ immune systems to induce rare precursor B cells of a class of HIV broadly neutralizing antibodies (bNAbs). The findings, published today in Nature Immunology, are an encouraging, incremental step in developing a preventive HIV vaccine.
High H5N1 Influenza Levels Found in Mice Given Raw Milk from Infected Dairy Cows
May 24, 2024
Mice administered raw milk samples from dairy cows infected with H5N1 influenza experienced high virus levels in their respiratory organs and lower virus levels in other vital organs, according to findings published in the New England Journal of Medicine. The results suggest that consumption of raw milk by animals poses a risk for H5N1 infection and raises questions about its potential risk in humans.
NIH Study Shows Chronic Wasting Disease Unlikely to Move from Animals to People
May 17, 2024
A new study of prion diseases, using a human cerebral organoid model, suggests there is a substantial species barrier preventing transmission of chronic wasting disease (CWD) from cervids—deer, elk and moose—to people. The findings, from National Institutes of Health scientists and published in Emerging Infectious Diseases, are consistent with decades of similar research in animal models at the NIH’s National Institute of Allergy and Infectious Diseases (NIAID).
Lower Dose of Mpox Vaccine Is Safe and Generates Six-Week Antibody Response Equivalent to Standard Regimen
April 27, 2024
A dose-sparing intradermal mpox vaccination regimen was safe and generated an antibody response equivalent to that induced by the standard regimen at six weeks (two weeks after the second dose), according to findings presented today at the European Society of Clinical Microbiology and Infectious Diseases Global Congress in Barcelona. The results suggest that antibody responses contributed to the effectiveness of dose-sparing mpox vaccine regimens used during the 2022 U.S. outbreak.
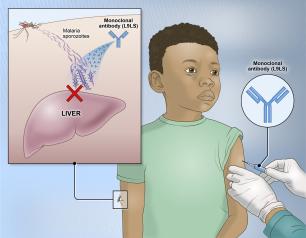
Experimental NIH Malaria Monoclonal Antibody Protective in Malian Children
April 26, 2024
One injected dose of an experimental malaria monoclonal antibody was 77% effective against malaria disease in children in Mali during the country’s six-month malaria season, according to the results of a mid-stage clinical trial. The trial assessed an investigational monoclonal antibody developed by scientists at the National Institutes of Health (NIH), and results appear in The New England Journal of Medicine.
World TB Day 2024 – Yes! We Can End TB!
March 22, 2024
In observance of World Tuberculosis Day (Sunday, March 24), NIAID joins our partners in reaffirming our commitment to ending the tuberculosis (TB) pandemic while honoring the lives lost to TB disease.
NIH Scientists Find Weak Points on Epstein-Barr Virus
March 12, 2024
Studies of interactions between two lab-generated monoclonal antibodies (mAbs) and an essential Epstein-Barr virus (EBV) protein have uncovered targets that could be exploited in designing treatments and vaccines for this extremely common virus. The research was led by Jeffrey I. Cohen, M.D., and colleagues from NIAID.
Long-Acting HIV Treatment Benefits Adults with Barriers to Daily Pill Taking and Adolescents with Suppressed HIV
March 6, 2024
Long-acting, injectable antiretroviral therapy (ART) suppressed HIV replication better than oral ART in people who had previously experienced challenges taking daily oral regimens and was found safe in adolescents with HIV viral suppression, according to two studies presented today at the 2024 Conference on Retroviruses and Opportunistic Infections (CROI) in Denver. Both studies were sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, in collaboration with other NIH institutes.

Children Surpass a Year of HIV Remission after Treatment Pause
March 6, 2024
Four children have remained free of detectable HIV for more than one year after their antiretroviral therapy (ART) was paused to see if they could achieve HIV remission, according to a presentation today at the 2024 Conference on Retroviruses and Opportunistic Infections (CROI) in Denver. The children, who acquired HIV before birth, were enrolled in a clinical trial funded by the National Institutes of Health in which an ART regimen was started within 48 hours of birth and then closely monitored for drug safety and HIV viral suppression.

Semaglutide Reduces Severity of Common Liver Disease in People with HIV
March 5, 2024
A weekly injection of semaglutide was safe and reduced the amount of fat in the liver by 31% in people with HIV and metabolic dysfunction-associated steatotic liver disease (MASLD), according to a presentation today at the 2024 Conference on Retroviruses and Opportunistic Infections (CROI) in Denver. This is the first clinical trial of semaglutide for MASLD in people with HIV.
Tools Underestimate Cardiovascular Event Risk in People with HIV
March 4, 2024
The elevated cardiovascular disease risk among people with HIV is even greater than predicted by a standard risk calculator in several groups, including Black people and cisgender women, according to analyses from a large international clinical trial primarily funded by the National institutes of Health and presented at the 2024 Conference on Retroviruses and Opportunistic Infections (CROI) in Denver.
New Antibodies Target “Dark Side” of Influenza Virus Protein
March 1, 2024
Researchers at the National Institutes of Health have identified antibodies targeting a hard-to-spot region of the influenza virus, shedding light on the relatively unexplored “dark side” of the neuraminidase (NA) protein head. The antibodies target a region of the NA protein that is common among many influenza viruses, including H3N2 subtype viruses, and could be a new target for countermeasures. The research, led by scientists at the National Institute of Allergy and Infectious Diseases’ Vaccine Research Center, part of NIH, was published today in Immunity.
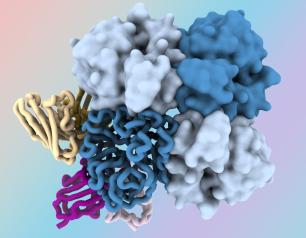
Statement: Long-Acting HIV Treatment Demonstrates Efficacy in People with Challenges Taking Daily Medicine as Prescribed
February 21, 2024
Long-acting antiretroviral therapy (ART) with cabotegravir and rilpivirine was superior in suppressing HIV replication compared to daily oral ART in people who had been unable to maintain viral suppression through an oral daily regimen, according to interim data from a randomized trial. Upon review of these findings, an independent Data and Safety Monitoring Board (DSMB) recommended halting randomization and inviting all eligible study participants to take long-acting ART.
COVID-19 Vaccination and Boosting During Pregnancy Protects Infants for Six Months
February 14, 2024
Women who receive an mRNA-based COVID-19 vaccination or booster during pregnancy can provide their infants with strong protection against symptomatic COVID-19 infection for at least six months after birth. These findings reinforce the importance of receiving both a COVID-19 vaccine and booster during pregnancy to ensure that infants are born with robust protection that lasts until they are old enough to be vaccinated.

Researchers Create Safer Form of Coxiella burnetii for Scientific Use
January 25, 2024
Scientists have unexpectedly discovered that the weakened form of the bacteria Coxiella burnetii (C. burnetii) not typically known to cause disease, naturally acquired an ability to do so. C. burnetii causes Q Fever in humans and its weakened forms are those used for scientific purposes. Subsequently, the scientists identified the genetic mutation responsible for the increased ability to cause disease (virulence) and created a form of the bacteria without the genetic flaw that could safely be used for research.
NIH-Developed HIV Antibodies Protect Animals in Proof-of-Concept Study
January 17, 2024
Three different HIV antibodies each independently protected monkeys from acquiring simian-HIV (SHIV) in a placebo-controlled proof-of-concept study intended to inform development of a preventive HIV vaccine for people. The antibodies—a human broadly neutralizing antibody and two antibodies isolated from previously vaccinated monkeys—target the fusion peptide, a site on an HIV surface protein that helps the virus fuse with and enter cells.
Biomedical STI Prevention Evidence Is Inadequate for Cisgender Women
December 20, 2023
Pivotal studies of some biomedical HIV and sexually transmitted infection (STI) prevention interventions have excluded cisgender women or demonstrated low efficacy among them, limiting their prevention options relative to other populations who experience high HIV and STI incidence. Findings show doxycycline postexposure prophylaxis (better known as DoxyPEP) did not prevent STI acquisition in cisgender women, despite showing promising results in gay, bisexual, and other men who have sex with men and transgender women in a previous study.
NIH Research Identifies Opportunities to Improve Future HIV Vaccine Candidates
December 14, 2023
An effective HIV vaccine may need to prompt strong responses from immune cells called CD8+ T cells to protect people from acquiring HIV, according to a new study from researchers at the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, and colleagues. The study findings, appearing in Science, draw comparisons between the immune system activity of past HIV vaccine study participants and people with HIV who naturally keep the virus from replicating even in the absence of antiretroviral therapy (ART).

NIH Clinical Trial of Tuberculous Meningitis Drug Regimen Begins
December 7, 2023
A trial of a new drug regimen to treat tuberculous meningitis (TBM) has started enrolling adults and adolescents in several countries where tuberculosis (TB) is prevalent. The trial will include 330 participants aged 15 years and older who have or are likely to have TBM based on signs and symptoms, including people living with and without HIV. Because pregnant women are eligible to enroll in this study with appropriate consent, a small number of pregnant women are expected to be included.