154 Results
NIH Clinical Trial of Tuberculous Meningitis Drug Regimen Begins
December 7, 2023
A trial of a new drug regimen to treat tuberculous meningitis (TBM) has started enrolling adults and adolescents in several countries where tuberculosis (TB) is prevalent. The trial will include 330 participants aged 15 years and older who have or are likely to have TBM based on signs and symptoms, including people living with and without HIV. Because pregnant women are eligible to enroll in this study with appropriate consent, a small number of pregnant women are expected to be included.
World AIDS Day 2023
December 1, 2023
On this 35th World AIDS Day, the National Institutes of Health (NIH) joins its partners in honoring the lives lost due to the HIV pandemic. For decades, this virus has exacted a tragic toll, affecting people, families, and communities worldwide, threatening social and economic development, and exacerbating stigma, often toward people who already experience discrimination and health disparities.

NIH Statement on Preliminary Efficacy Results of First-in-Class Gonorrhea Antibiotic Developed Through Public-Private Partnership
November 1, 2023
A single dose of a novel oral antibiotic called zoliflodacin has been found to be as safe and effective as standard therapy for uncomplicated urogenital gonorrhea in an international Phase 3 non-inferiority clinical trial. Gonorrhea treatment options are increasingly limited due to antimicrobial resistance seen in Neisseria gonorrhoeae, the bacteria that cause gonococcal infection.

Study Reveals How Young Children’s Immune Systems Tame SARS-CoV-2
October 13, 2023
A study of infants and young children found those who acquired SARS-CoV-2 had a strong, sustained antibody response to the virus and high levels of inflammatory proteins in the nose but not in the blood. This immune response contrasts with that typically seen in adults with SARS-CoV-2 infection.
Clinical Trial to Test Immune Modulation Strategy for Hospitalized COVID-19 Patients Begins
September 22, 2023
A clinical trial has launched to test whether early intensive immune modulation for hospitalized COVID-19 patients with relatively mild illness is beneficial. The placebo-controlled study will enroll approximately 1,500 people at research sites around the world.
Clinical Trial of HIV Vaccine Begins in United States and South Africa
September 20, 2023
A trial of a preventive HIV vaccine candidate has begun enrollment in the United States and South Africa. The Phase 1 trial will evaluate a novel vaccine known as VIR-1388 for its safety and ability to induce an HIV-specific immune response in people.
NIH Releases Strategic Plan for Research on Herpes Simplex Virus 1 and 2
September 19, 2023
In response to the persistent health challenges of herpes simplex virus 1 (HSV-1) and HSV-2, an NIH-wide HSV Working Group developed the plan, informed by feedback from more than 100 representatives of the research and advocacy communities and interested public stakeholders. The plan outlines an HSV research framework with four strategic priorities: improving fundamental knowledge of HSV biology, pathogenesis, and epidemiology; accelerating research to improve HSV diagnosis; improving strategies to treat HSV while seeking a curative therapeutic; and, advancing research to prevent HSV infection.
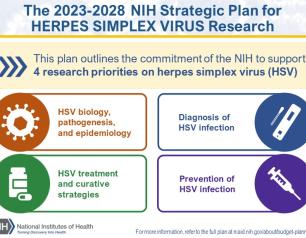
NIH Clinical Trial of Universal Flu Vaccine Candidate Begins
September 15, 2023
Enrollment in a Phase 1 trial of a new investigational universal influenza vaccine candidate has begun at the National Institutes of Health’s Clinical Center in Bethesda, Maryland. The trial is sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the NIH, and will evaluate the investigational vaccine for safety and its ability to elicit an immune response.
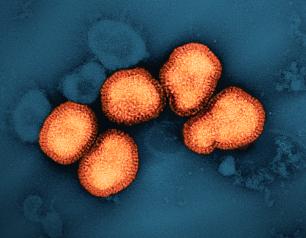
NIH Investigates Multidrug-Resistant Bacterium Emerging in Community Settings
September 6, 2023
New “hypervirulent” strains of the bacterium Klebsiella pneumoniae have emerged in healthy people in community settings, prompting a National Institutes of Health research group to investigate how the human immune system defends against infection.
NIH Study Examines Connections Between Drinking Water Quality and Increased Lung Infections in People with Cystic Fibrosis
August 25, 2023
High levels of some minerals and metals in environmental water supplies may increase the risk of nontuberculous mycobacteria (NTM) pulmonary infections in people with cystic fibrosis. The study appearing in Environmental Epidemiology, found the presence of the metals molybdenum and vanadium along with sulfate—a collection of mineral salts—in the U.S. municipal water system was associated with an increased incidence of NTM pulmonary infections, the leading cause of drinking-water associated illnesses.
Severe COVID-19 May Lead to Long-Term Innate Immune System Changes
August 18, 2023
Severe COVID-19 may cause long-lasting alterations to the innate immune system, the first line of defense against pathogens, according to a small study funded by the National Institute of Allergy and Infectious Diseases.
COVID-19 Vaccination and Boosting During Pregnancy Benefits Pregnant People and Newborns
August 11, 2023
Receiving a COVID-19 mRNA vaccine or booster during pregnancy can benefit pregnant people and their newborn infants, according to findings recently published in Vaccine. The paper describes results from the Multisite Observational Maternal and Infant Study for COVID-19 (MOMI-VAX), which was funded by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health.
Daily Statin Reduces Heart Disease Risk Among Adults Living with HIV
July 24, 2023
A National Institutes of Health-supported study found that statins, a class of cholesterol-lowering medications, may offset the high risk of cardiovascular disease in people living with HIV by more than a third, potentially preventing one in five major cardiovascular events or premature deaths in this population. People living with HIV can have a 50-100% increased risk for cardiovascular disease.
Investigational Three-Month TB Regimen Is Safe but Ineffective, NIH Study Finds
July 5, 2023
The first clinical trial of a three-month tuberculosis (TB) treatment regimen is closing enrollment because of a high rate of unfavorable outcomes with the investigational course of treatment. Advancing Clinical Therapeutics Globally for HIV/AIDS and Other Infections (ACTG) 5362, also known as the CLO-FAST trial, sought to evaluate the safety and efficacy of a three-month clofazimine- and high-dose rifapentine-containing regimen. An interim data analysis showed that participants taking the investigational regimen experienced ongoing or recurring TB at rates above thresholds set in the study protocol.
NIH Statement on HIV Vaccine Awareness Day 2023
May 18, 2023
Today marks the 26th observance of HIV Vaccine Awareness Day. The National Institutes of Health applauds the efforts of the collaborative global community of scientists, advocates, study participants, study staff, and funders enabling unprecedented levels of innovation and adaptation in the pursuit of a highly effective HIV vaccine.
First-in-Human Trial of Oral Drug to Remove Radioactive Contamination Begins
May 15, 2023
A first-in-human clinical trial of an experimental oral drug for removing radioactive contaminants from inside the body has begun. The trial is testing the safety, tolerability and processing in the body of escalating doses of the investigational drug product HOPO 14-1 in healthy adults. The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, is funding the Phase 1 trial, which is sponsored and conducted by SRI International of Menlo Park, California.

Clinical Trial of mRNA Universal Influenza Vaccine Candidate Begins
May 15, 2023
A clinical trial of an experimental universal influenza vaccine developed by researchers at the National Institute of Allergy and Infectious Diseases’ (NIAID) Vaccine Research Center (VRC), part of the National Institutes of Health, has begun enrolling volunteers at Duke University in Durham, North Carolina. This Phase 1 trial will test the experimental vaccine, known as H1ssF-3928 mRNA-LNP, for safety and its ability to induce an immune response.
NIH Celebrates FDA Approval of RSV Vaccine for People 60 Years of Age and Older
May 4, 2023
Food and Drug Administration announced the approval of the first respiratory syncytial virus (RSV) vaccine approved for use in the United States. The vaccine, Arexvy, is approved for the prevention of lower respiratory tract disease caused by RSV in individuals 60 years of age and older.
Study Shows Most Children Recover from Lyme Disease within Six Months of Treatment
April 21, 2023
A majority of parents of children diagnosed with Lyme disease reported that their kids recovered within six months of completing antibiotic treatment, according to a new joint study from Children’s National Research Institute and the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, published in Pediatric Research.

The Potential and Challenges of Mucosal COVID-19 Vaccines
April 13, 2023
In November 2022, the National Institute of Allergy and Infectious Diseases (NIAID) co-hosted a virtual workshop on the importance and challenges of developing mucosal vaccines for SARS-COV-2. The highlights of this workshop have now been published as a report in npj Vaccines.
Daily Statin Reduces the Risk of Cardiovascular Disease in People Living with HIV, Large NIH Study Finds
April 11, 2023
A National Institutes of Health (NIH) clinical trial was stopped early because a daily statin medication was found to reduce the increased risk of cardiovascular disease among people living with HIV in the first large-scale clinical study to test a primary cardiovascular prevention strategy in this population.
NIH-Funded Study Finds Doxycycline Reduces Sexually Transmitted Infections by Two-Thirds
April 6, 2023
The oral antibiotic doxycycline prevented the acquisition of sexually transmitted infections (STIs) when tested among study participants who took the medication within 72 hours of having condomless sex. The post-exposure approach, termed doxy-PEP, resulted in a two-thirds reduction in the incidence of syphilis, gonorrhea, and chlamydia among the study participants.
World TB Day 2023 – ‘Yes! We Can End TB!’
March 24, 2023
Each year, on March 24, the National Institute of Allergy and Infectious Diseases (NIAID), part of the NIH, joins people and organizations from around the globe in marking World Tuberculosis Day. On this day, more than 140 years ago, Dr. Robert Koch announced his discovery that most human tuberculosis (TB) is caused by the bacterium Mycobacterium tuberculosis (Mtb). Although our scientific insight into this disease has grown over the past century, TB is still one of the deadliest infectious diseases on the planet. Today, NIAID joins the world in a message of hope: “Yes!
SARS-CoV-2 Infection Weakens Immune-Cell Response to Vaccination
March 20, 2023
The magnitude and quality of a key immune cell’s response to vaccination with two doses of the Pfizer-BioNTech COVID-19 vaccine were considerably lower in people with prior SARS-CoV-2 infection compared to people without prior infection, a study has found. In addition, the level of this key immune cell that targets the SARS-CoV-2 spike protein was substantially lower in unvaccinated people with COVID-19 than in vaccinated people who had never been infected. Importantly, people who recover from SARS-CoV-2 infection and then get vaccinated are more protected than people who are unvaccinated.
Temperature-Stable TB Vaccine Safe, Prompts Immune Response in NIH-Supported Study
March 6, 2023
A clinical trial testing a freeze-dried, temperature-stable experimental tuberculosis (TB) vaccine in healthy adults found that it was safe and stimulated both antibodies and responses from the cellular arm of the immune system. The Phase 1 trial was supported by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health. A non-temperature stable form of the candidate previously had been tested in several clinical trials.