154 Results
NIH Trial to Evaluate Shionogi Antiviral in Adults Hospitalized with COVID-19
February 15, 2023
The National Institutes of Health has initiated a multi-site clinical trial evaluating an investigational antiviral for the treatment of COVID-19. The therapeutic, known as S-217622 or ensitrelvir fumaric acid, was discovered by Hokkaido University, Sapporo, Japan; and Shionogi & Co., Ltd., Osaka, Japan. The trial is assessing whether S-217622 can improve clinical outcomes for patients who are hospitalized for management of COVID-19 as compared to a placebo and will enroll approximately 1,500 people at sites worldwide.
NIH Scientists Develop Mouse Model to Study Mpox Virulence
February 14, 2023
Scientists from the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, have removed a major roadblock to better understanding of mpox (formerly, monkeypox). They developed a mouse model of the disease and used it to demonstrate clear differences in virulence among the major genetic groups (clades) of mpox virus (MPXV). The research, appearing in Proceedings of the National Academy of Science, was led by Bernard Moss, M.D., Ph.D., chief of the Genetic Engineering Section of NIAID’s Laboratory of Viral Diseases.
Experimental NIH Sudan Virus Vaccine Protects Macaques
February 2, 2023
A National Institutes of Health research group with extensive experience studying ebolavirus countermeasures has successfully developed a vaccine against Sudan virus (SUDV) based on the licensed Ebola virus (EBOV) vaccine. SUDV, identified in 1976, is one of the four viruses known to cause human Ebolavirus disease. The new vaccine, VSV-SUDV, completely protected cynomolgus macaques against a lethal SUDV challenge. The findings were published in the journal The Lancet Microbe.
Marburg Vaccine Shows Promising Results in First-in-Human Study
January 30, 2023
A newly published paper in The Lancet shows that an experimental vaccine against Marburg virus (MARV) was safe and induced an immune response in a small, first-in-human clinical trial. The vaccine, developed by researchers at the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, could someday be an important tool to respond to Marburg virus outbreaks.
Experimental HIV Vaccine Regimen Safe but Ineffective, NIH Study Finds
January 18, 2023
An investigational HIV vaccine regimen tested among men who have sex with men (MSM) and transgender people was safe but did not provide protection against HIV acquisition, an independent data and safety monitoring board (DSMB) has determined. The HPX3002/HVTN 706, or “Mosaico,” Phase 3 clinical trial began in 2019 and involved 3,900 volunteers ages 18 to 60 years in Europe, North America and South America.
Developing Mucosal Vaccines for Respiratory Viruses
January 11, 2023
Vaccines that provide long-lasting protection against influenza, coronaviruses and respiratory syncytial virus (RSV) have proved exceptionally difficult to develop. In a new review article in Cell Host & Microbe, researchers from the National Institute of Allergy and Infectious Diseases (NIAID), part of the NIH, explore the challenges and outline approaches to improved vaccines. Anthony S. Fauci, M.D., former NIAID director, is an author along with Jeffery K. Taubenberger, M.D., Ph.D., and David M. Morens, M.D.
Ebola Vaccine Regimens Safe, Immunogenic in Adults and Children
December 14, 2022
Two randomized, placebo-controlled trials evaluating three Ebola vaccine administration strategies in adults and children found that all the regimens were safe in both age groups, according to results published today in the New England Journal of Medicine. Antibodies were produced in response to the vaccine regimens beginning at 14 days after the first vaccination and continued to be detectable at varying levels—depending on the vaccine and regimen used—in both children and adults for one year.
Dr. Fauci Reflects on the Perpetual Challenge of Infectious Diseases
November 28, 2022
Dr. Fauci, who since 1984 has directed the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, reflects on his career responding to infectious disease threats.

NIH Awards $12 Million for Antiviral Therapeutic Development
November 21, 2022
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, recently awarded more than $12 million to three institutions for the development of antiviral therapies to treat diseases caused by viruses with pandemic potential.
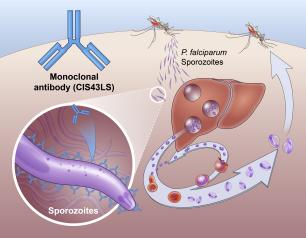
Monoclonal Antibody Prevents Malaria Infection in African Adults
October 31, 2022
One dose of an antibody drug safely protected healthy, non-pregnant adults from malaria infection during an intense six-month malaria season in Mali, Africa, a National Institutes of Health clinical trial has found. The antibody was up to 88.2% effective at preventing infection over a 24-week period, demonstrating for the first time that a monoclonal antibody can prevent malaria infection in an endemic region.
Experimental Monoclonal Antibodies Show Promise Against Epstein-Barr Virus
October 27, 2022
A panel of investigational monoclonal antibodies (mAbs) targeting different sites of the Epstein-Barr virus (EBV) blocked infection when tested in human cells in a laboratory setting. Moreover, one of the experimental mAbs provided nearly complete protection against EBV infection and lymphoma when tested in mice. The results appear online in the journal Immunity.
Monkeypox Treatment Trial Begins in the Democratic Republic of the Congo
October 12, 2022
A clinical trial to evaluate the antiviral drug tecovirimat, also known as TPOXX, in adults and children with monkeypox has begun in the Democratic Republic of the Congo (DRC). The trial will evaluate the drug’s safety and its ability to mitigate monkeypox symptoms and prevent serious outcomes, including death. NIAID and the DRC’s National Institute for Biomedical Research (INRB) are co-leading the trial as part of the government-to-government PALM partnership. Collaborating institutions include the U.S. Centers for Disease Control and Prevention (CDC), the Institute of Tropical Medicine Antwerp, the aid organization Alliance for International Medical Action (ALIMA) and the World Health Organization (WHO).
Findings Suggest COVID-19 Rebound Not Caused by Impaired Immune Response
October 6, 2022
Findings from a small study of eight patients published in Clinical Infectious Diseases suggest that COVID-19 rebound is likely not caused by impaired immune responses. The study, led by scientists at the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, aimed to define the clinical course and the immunologic and virologic characteristics of COVID-19 rebound in patients who have taken nirmatrelvir/ritonavir (Paxlovid), an antiviral therapeutic developed by Pfizer, Inc.

NIH-Supported Clinical Trial of Phage Therapy for Cystic Fibrosis Begins
October 4, 2022
Enrollment has begun in an early-stage clinical trial evaluating bacteriophage therapy in adults with cystic fibrosis (CF) who carry Pseudomonas aeruginosa (P. aeruginosa) in their lungs. The trial is evaluating whether the bacteriophage, or “phage,” therapy is safe and able to reduce the amount of bacteria in the lungs of volunteers. The trial is being conducted by the Antibacterial Resistance Leadership Group (ARLG), funded by the National Institute of Allergy and Infectious Diseases.
U.S. Clinical Trial Evaluating Antiviral for Monkeypox Begins
September 9, 2022
A Phase 3 clinical trial evaluating the antiviral tecovirimat, also known as TPOXX, is now enrolling adults and children with monkeypox infection in the United States. Study investigators aim to enroll more than 500 people from clinical research sites nationwide. Interested volunteers can visit the ACTG website (clinical trial A5418) for more information. The NIAID-funded Advancing Clinical Therapeutics Globally for HIV/AIDS and Other Infections (ACTG) is leading the study, which may later expand to international sites. The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) at NIH is supporting several sites, including through the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT).
Clinical Trial Evaluating Monkeypox Vaccine Begins
September 8, 2022
A clinical trial evaluating alternative strategies for administering the JYNNEOS monkeypox vaccine to increase the number of available doses has begun enrolling adult volunteers. The trial, which will enroll more than 200 adults across eight U.S. research sites, is sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health. JYNNEOS is manufactured by Bavarian Nordic, based in Copenhagen. It is approved by the U.S.
SARS-CoV-2 Antigen Levels Linked to Patient Outcomes
August 29, 2022
The amount of SARS-CoV-2 antigen measured in the blood of patients hospitalized with COVID-19 is associated with illness severity and other clinical outcomes, according to a new study published in the Annals of Internal Medicine. The authors suggest that SARS-CoV-2 antigen levels hold promise as a biomarker, or a measurable substance, to predict which patients hospitalized with COVID-19 have a higher risk of worse outcomes.
NIH Experts Review Monkeypox Challenges
August 24, 2022
National Institutes of Health experts write that lessons learned from the public health responses to the HIV and COVID-19 pandemics should help guide the response to the current outbreak of monkeypox.
Monoclonal Antibody Prevents Malaria in U.S. Adults, NIH Trial Shows
August 4, 2022
One injection of a candidate monoclonal antibody (mAb) known as L9LS was found to be safe and highly protective in U.S. adults exposed to the malaria parasite, according to results from a National Institutes of Health Phase 1 clinical trial published in The New England Journal of Medicine.
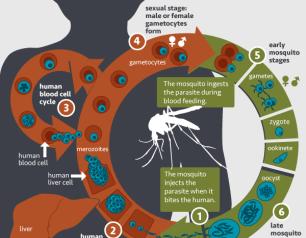
New Insights into HIV Latent Cells Yield Potential Cure Targets
July 27, 2022
Scientists with the National Institute of Allergy and Infectious Diseases’ (NIAID) Vaccine Research Center (VRC) and their collaborators described how their use of cutting-edge technology revealed new insights into cellular reservoirs of HIV and what those observations could mean for the next steps in HIV cure research.
Statement from NIH and BARDA on the Novavax COVID-19 Vaccine
July 20, 2022
The Centers for Disease Control and Prevention (CDC) has recommended that Novavax’s COVID-19 vaccine be used as another primary series option for adults in the United States ages 18 years and older. The Food and Drug Administration (FDA) previously authorized for emergency use the protein-based vaccine, known as NVX-CoV2373.
NIH Launches Clinical Trial of mRNA Nipah Virus Vaccine
July 11, 2022
The National Institute of Allergy and Infectious Diseases has launched an early-stage clinical trial evaluating an investigational vaccine to prevent infection with Nipah virus.
Trial of Potential Universal Flu Vaccine Opens at NIH Clinical Center
June 28, 2022
A Phase 1 clinical trial of a novel influenza vaccine has begun inoculating healthy adult volunteers at the National Institutes of Health Clinical Center in Bethesda, Maryland. The placebo-controlled trial will test the safety of a candidate vaccine, BPL-1357, and its ability to prompt immune responses.
Food Allergy Is Associated with Lower Risk of SARS-CoV-2 Infection
June 1, 2022
A National Institutes of Health-funded study has found that people with food allergies are less likely to become infected with SARS-CoV-2, the virus that causes COVID-19, than people without them.
Combination Anti-HIV Antibody Infusions Suppress Virus for Prolonged Period
June 1, 2022
According to a small study published today in the journal Nature, individuals with HIV who began taking antiretroviral therapy (ART) in the early stages of infection achieved a lengthy period of HIV suppression without ART after receiving two broadly neutralizing anti-HIV antibodies (bNAbs).