40 Results
NIH-Sponsored Trial of Lassa Vaccine Opens
March 17, 2025
An NIAID-sponsored clinical trial of an experimental vaccine to prevent Lassa fever has begun.
NIH Study Finds Tecovirimat Was Safe but Did Not Improve Mpox Resolution or Pain
December 10, 2024
Tecovirimat was safe but did not reduce the time to lesion resolution or reduce pain among adults with mild to moderate clade II mpox and a low risk of severe disease in an international study.
NIH Statement on World AIDS Day
December 2, 2024
Together with our partners, the National Institutes of Health (NIH) commemorates World AIDS Day and affirms our commitment to bolstering the extraordinary gains achieved in HIV science and to persevering until we end HIV-related illness and stigma. As we mark this observance, we celebrate the people who enable scientific progress, honor the loved ones and leaders we have lost, and reflect on the work that remains to ensure the health and life quality of all people affected by, and living with, HIV.
NIH Releases Mpox Research Agenda
September 17, 2024
The NIAID mpox research agenda focuses on four key objectives: increasing knowledge about the biology of all clades—also known as strains—of the virus that causes mpox, including how the virus is transmitted and how people’s immune systems respond to it; evaluating dosing regimens of current vaccines to stretch the vaccine supply and developing novel vaccine concepts; advancing existing and novel treatments, including antivirals and monoclonal antibodies; and supporting strategies for detecting the virus to facilitate clinical care and epidemiological surveillance.

The Antiviral Tecovirimat is Safe but Did Not Improve Clade I Mpox Resolution in Democratic Republic of the Congo
August 15, 2024
The antiviral drug tecovirimat did not reduce the duration of mpox lesions among children and adults with clade I mpox in the Democratic Republic of the Congo (DRC), based on an initial analysis of data from a randomized, placebo-controlled trial.
Candidate Malaria Vaccine Provides Lasting Protection in NIH-Sponsored Trials
August 14, 2024
Two National Institutes of Health (NIH)-supported trials of an experimental malaria vaccine in healthy Malian adults found that all three tested regimens were safe.
An Isolated Viral Load Test May Generate False Positive Results for People Using Long-Acting PrEP
July 23, 2024
A single laboratory-based HIV viral load test used by U.S. clinicians who provide people with long-acting, injectable cabotegravir (CAB-LA) HIV pre-exposure prophylaxis (PrEP) did not reliably detect HIV in a multi-country study. In the study, a single positive viral load test was frequently found to be a false positive result.
Long-Acting Injectable Cabotegravir for HIV Prevention Is Safe in Pregnancy
July 23, 2024
Long-acting injectable cabotegravir (CAB-LA) was safe and well tolerated as HIV pre-exposure prophylaxis (PrEP) before and during pregnancy in the follow-up phase of a global study among cisgender women.
Exploratory Analysis Associates HIV Drug Abacavir with Elevated Cardiovascular Disease Risk in Large Global Trial
July 23, 2024
Current or previous use of the antiretroviral drug (ARV) abacavir was associated with an elevated risk of major adverse cardiovascular events (MACE) in people with HIV, according to an exploratory analysis from a large international clinical trial primarily funded by the National Institutes of Health (NIH).
NIH Statement on Preliminary Efficacy Results of Twice-Yearly Lenacapavir for HIV Prevention in Cisgender Women
June 26, 2024
The injectable antiretroviral drug lenacapavir was safe and 100% effective as long-acting HIV pre-exposure prophylaxis (PrEP) among cisgender women in a Phase 3 clinical trial, according to top-line findings released by Gilead Sciences, Inc., the study sponsor. Lenacapavir is administered every six months, making it the most durable HIV prevention method to have shown efficacy in this population.
Lower Dose of Mpox Vaccine Is Safe and Generates Six-Week Antibody Response Equivalent to Standard Regimen
April 27, 2024
A dose-sparing intradermal mpox vaccination regimen was safe and generated an antibody response equivalent to that induced by the standard regimen at six weeks (two weeks after the second dose), according to findings presented today at the European Society of Clinical Microbiology and Infectious Diseases Global Congress in Barcelona. The results suggest that antibody responses contributed to the effectiveness of dose-sparing mpox vaccine regimens used during the 2022 U.S. outbreak.
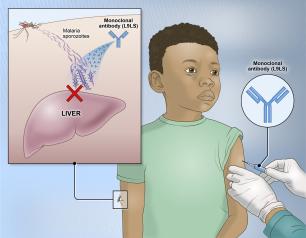
Experimental NIH Malaria Monoclonal Antibody Protective in Malian Children
April 26, 2024
One injected dose of an experimental malaria monoclonal antibody was 77% effective against malaria disease in children in Mali during the country’s six-month malaria season, according to the results of a mid-stage clinical trial. The trial assessed an investigational monoclonal antibody developed by scientists at the National Institutes of Health (NIH), and results appear in The New England Journal of Medicine.
World TB Day 2024 – Yes! We Can End TB!
March 22, 2024
In observance of World Tuberculosis Day (Sunday, March 24), NIAID joins our partners in reaffirming our commitment to ending the tuberculosis (TB) pandemic while honoring the lives lost to TB disease.
Children Surpass a Year of HIV Remission after Treatment Pause
March 6, 2024
Four children have remained free of detectable HIV for more than one year after their antiretroviral therapy (ART) was paused to see if they could achieve HIV remission, according to a presentation today at the 2024 Conference on Retroviruses and Opportunistic Infections (CROI) in Denver.

Long-Acting HIV Treatment Benefits Adults with Barriers to Daily Pill Taking and Adolescents with Suppressed HIV
March 6, 2024
Long-acting, injectable antiretroviral therapy (ART) suppressed HIV replication better than oral ART in people who had previously experienced challenges taking daily oral regimens and was found safe in adolescents with HIV viral suppression, according to two studies presented today at the 2024 Conference on Retroviruses and Opportunistic Infections (CROI) in Denver. Both studies were sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, in collaboration with other NIH institutes.

Vaginal Ring and Oral Pre-Exposure Prophylaxis Found Safe for HIV Prevention Throughout Pregnancy
March 5, 2024
The monthly dapivirine vaginal ring and daily oral pre-exposure prophylaxis (PrEP) with tenofovir disoproxil fumarate and emtricitabine were each found to be safe for HIV prevention among cisgender women who started using one of them in their second trimester of pregnancy, according to findings presented today at the 2024 Conference on Retroviruses and Opportunistic Infections (CROI) in Denver.
Biomedical STI Prevention Evidence Is Inadequate for Cisgender Women
December 20, 2023
Pivotal studies of some biomedical HIV and sexually transmitted infection (STI) prevention interventions have excluded cisgender women or demonstrated low efficacy among them, limiting their prevention options relative to other populations who experience high HIV and STI incidence. Findings show doxycycline postexposure prophylaxis (better known as DoxyPEP) did not prevent STI acquisition in cisgender women, despite showing promising results in gay, bisexual, and other men who have sex with men and transgender women in a previous study.
Investigational Three-Month TB Regimen Is Safe but Ineffective, NIH Study Finds
July 5, 2023
The first clinical trial of a three-month tuberculosis (TB) treatment regimen is closing enrollment because of a high rate of unfavorable outcomes with the investigational course of treatment. Advancing Clinical Therapeutics Globally for HIV/AIDS and Other Infections (ACTG) 5362, also known as the CLO-FAST trial, sought to evaluate the safety and efficacy of a three-month clofazimine- and high-dose rifapentine-containing regimen. An interim data analysis showed that participants taking the investigational regimen experienced ongoing or recurring TB at rates above thresholds set in the study protocol.
NIAID Marks World Malaria Day
April 25, 2023
World Malaria Day is an opportunity to reflect on continuing challenges posed by malaria and reaffirm a commitment to overcoming them. The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, joins with the global health community in recognizing this year’s theme of “Time to Deliver on Zero Malaria: Invest, Innovate, Implement.”
World TB Day 2023 – ‘Yes! We Can End TB!’
March 24, 2023
Each year, on March 24, the National Institute of Allergy and Infectious Diseases (NIAID), part of the NIH, joins people and organizations from around the globe in marking World Tuberculosis Day. On this day, more than 140 years ago, Dr. Robert Koch announced his discovery that most human tuberculosis (TB) is caused by the bacterium Mycobacterium tuberculosis (Mtb). Although our scientific insight into this disease has grown over the past century, TB is still one of the deadliest infectious diseases on the planet. Today, NIAID joins the world in a message of hope: “Yes!
Ebola Vaccine Regimens Safe, Immunogenic in Adults and Children
December 14, 2022
Two randomized, placebo-controlled trials evaluating three Ebola vaccine administration strategies in adults and children found that all the regimens were safe in both age groups, according to results published today in the New England Journal of Medicine. Antibodies were produced in response to the vaccine regimens beginning at 14 days after the first vaccination and continued to be detectable at varying levels—depending on the vaccine and regimen used—in both children and adults for one year.
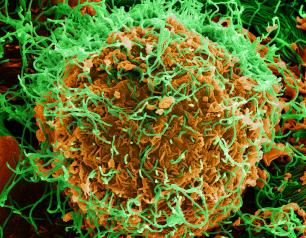
Monoclonal Antibody Prevents Malaria Infection in African Adults
October 31, 2022
One dose of an antibody drug safely protected healthy, non-pregnant adults from malaria infection during an intense six-month malaria season in Mali, Africa, a National Institutes of Health clinical trial has found. The antibody was up to 88.2% effective at preventing infection over a 24-week period, demonstrating for the first time that a monoclonal antibody can prevent malaria infection in an endemic region.
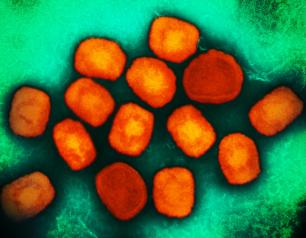
Monkeypox Treatment Trial Begins in the Democratic Republic of the Congo
October 12, 2022
A clinical trial to evaluate the antiviral drug tecovirimat, also known as TPOXX, in adults and children with monkeypox has begun in the Democratic Republic of the Congo (DRC). The trial will evaluate the drug’s safety and its ability to mitigate monkeypox symptoms and prevent serious outcomes, including death. NIAID and the DRC’s National Institute for Biomedical Research (INRB) are co-leading the trial as part of the government-to-government PALM partnership. Collaborating institutions include the U.S. Centers for Disease Control and Prevention (CDC), the Institute of Tropical Medicine Antwerp, the aid organization Alliance for International Medical Action (ALIMA) and the World Health Organization (WHO).
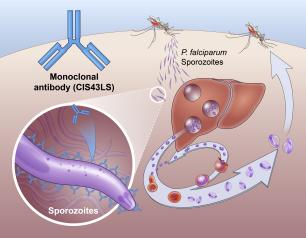
Monoclonal Antibody Prevents Malaria in U.S. Adults, NIH Trial Shows
August 4, 2022
One injection of a candidate monoclonal antibody (mAb) known as L9LS was found to be safe and highly protective in U.S. adults exposed to the malaria parasite, according to results from a National Institutes of Health Phase 1 clinical trial published in The New England Journal of Medicine.
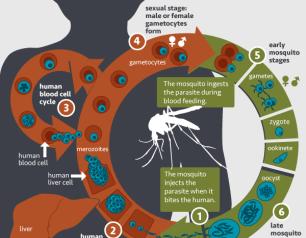
NIAID Marks World Malaria Day
April 25, 2022
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), reaffirms our commitment to developing safe and effective medical tools against malaria, and, in the words of this year’s World Malaria Day theme, “Harnessing innovation to reducing the malaria disease burden and saving lives.”

