66 Results
Statement from NIH and BARDA on the FDA Emergency Use Authorization of the Janssen COVID-19 Vaccine
February 27, 2021
Today, the U.S. Food and Drug Administration issued an Emergency Use Authorization (EUA) to the Janssen Pharmaceuticals Companies of Johnson & Johnson for its single-shot COVID-19 vaccine, called Ad.26.COV2S or JNJ-78436725. The Janssen vaccine is a recombinant vector vaccine that uses a human adenovirus to express the spike protein found on the surface of the SARS-CoV-2 virus that causes COVID-19.
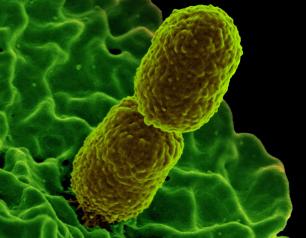
Mouse Study Shows Bacteriophage Therapy Could Fight Drug-Resistant Klebsiella pneumoniae
February 23, 2021
NIH study finds using viruses instead of antibiotics to tame drug-resistant bacteria is a promising strategy, known as bacteriophage or “phage therapy.”
Monoclonal Antibodies Against MERS Coronavirus Show Promise in Phase 1 NIH-Sponsored Trial
February 23, 2021
A NIH-sponsored Phase 1 clinical trial of two mAbs directed against the coronavirus that causes MERS found they were well tolerated and generally safe.
To End HIV Epidemic, We Must Address Health Disparities
February 19, 2021
NIH reports that scientific strides in HIV treatment and prevention have reduced transmissions and HIV-related deaths significantly in the US.
Statement—Four Potential COVID-19 Therapeutics Enter Phase 2/3 Testing in NIH ACTIV-2 Trial
February 12, 2021
Enrollment has begun to test additional investigational drugs in the NIH Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) program.
NIH Experts Discuss SARS-CoV-2 Viral Variants
February 12, 2021
The rise of several significant variants of SARS-CoV-2 has attracted the attention of health and science experts worldwide, NIH reports.
Clinical Trial in Hospitalized COVID-19 Patients Evaluates Long-Acting Antibody Therapy
February 8, 2021
A NIAID clinical trial began evaluating the safety of an investigational long-acting antibody combination for people hospitalized with COVID-19.
Intranasal Influenza Vaccine Spurs Strong Immune Response in Phase 1 Study
February 3, 2021
An experimental influenza vaccine was safe and produced a durable immune response when tested in a Phase 1 study, NIH reports.
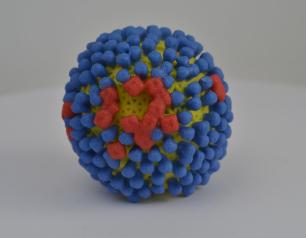
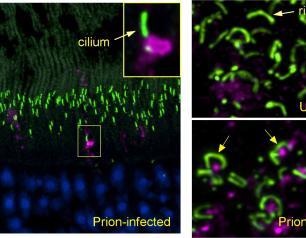
Scientists Identify Locations of Early Prion Protein Deposition in Retina
January 29, 2021
The earliest eye damage from prion disease takes place in the cone photoreceptor cells according to a new NIH study of prion protein accumulation.
Statement—Janssen Investigational COVID-19 Vaccine—Interim Analysis
January 29, 2021
NIH reports that an investigational COVID-19 vaccine by Janssen Pharmaceuticals appears to be safe and effective at preventing COVID-19 in adults.
Bulletin—Update on SARS-CoV-2 Variants
January 27, 2021
Recently, NIH notes that media reports and pre-print scientific papers on SARS-CoV-2 variants have discussed various genetic mutations in the virus.
Antibody Infusions Prevent Acquisition of Some HIV Strains, NIH Studies Find
January 26, 2021
NIH finds that an investigational anti-HIV antibody prevented acquisition of some HIV strains, but did not significantly reduce overall acquisition.
Media Availability—NIH Officials Highlight COVID-19 Vaccine Facts, Unknowns for Healthcare Providers
January 18, 2021
NIAID Director urges healthcare providers to be able to explain the latest data supporting the safety and efficacy of vaccines for COVID-19.
NIH Scientists Identify Nutrient that Helps Prevent Bacterial Infection
January 15, 2021
NIAID scientists studying natural defenses against bacterial infection found that taurine helps the gut recall prior infections and kill invading bacteria.
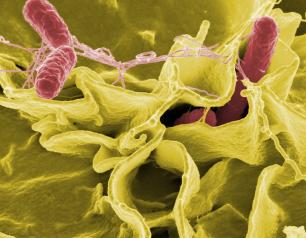
NIH Scientists Study Salmonella Swimming Behavior as Clues to Infection
January 13, 2021
NIH scientists believe they have identified a protein, that allows the Salmonella bacteria to swim straight when they are ready to infect cells.
Statement—Large Clinical Trial Will Test Combination Monoclonal Antibody Therapy for Mild/Moderate COVID-19
January 5, 2021
A NIAID-supported clinical trial has begun to evaluate a combination investigational monoclonal antibody therapy for people with mild to moderate COVID-19.