45 Results
Biomedical STI Prevention Evidence Is Inadequate for Cisgender Women
December 20, 2023
Pivotal studies of some biomedical HIV and sexually transmitted infection (STI) prevention interventions have excluded cisgender women or demonstrated low efficacy among them, limiting their prevention options relative to other populations who experience high HIV and STI incidence. Findings show doxycycline postexposure prophylaxis (better known as DoxyPEP) did not prevent STI acquisition in cisgender women, despite showing promising results in gay, bisexual, and other men who have sex with men and transgender women in a previous study.
Statement: Antibody Reduces Allergic Reactions to Multiple Foods in NIH Trial
December 19, 2023
A monoclonal antibody treatment significantly increased the amounts of multiple common foods that food-allergic children and adolescents could consume without an allergic reaction, according to a planned interim analysis of an advanced clinical trial.

NIH Research Identifies Opportunities to Improve Future HIV Vaccine Candidates
December 14, 2023
An effective HIV vaccine may need to prompt strong responses from immune cells called CD8+ T cells to protect people from acquiring HIV, according to a new study from researchers at the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, and colleagues. The study findings, appearing in Science, draw comparisons between the immune system activity of past HIV vaccine study participants and people with HIV who naturally keep the virus from replicating even in the absence of antiretroviral therapy (ART).

NIH Clinical Trial of Tuberculous Meningitis Drug Regimen Begins
December 7, 2023
A trial of a new drug regimen to treat tuberculous meningitis (TBM) has started enrolling adults and adolescents in several countries where tuberculosis (TB) is prevalent. The trial will include 330 participants aged 15 years and older who have or are likely to have TBM based on signs and symptoms, including people living with and without HIV. Because pregnant women are eligible to enroll in this study with appropriate consent, a small number of pregnant women are expected to be included.
World AIDS Day 2023
December 1, 2023
On this 35th World AIDS Day, the National Institutes of Health (NIH) joins its partners in honoring the lives lost due to the HIV pandemic. For decades, this virus has exacted a tragic toll, affecting people, families, and communities worldwide, threatening social and economic development, and exacerbating stigma, often toward people who already experience discrimination and health disparities.

NIH Statement on Preliminary Efficacy Results of First-in-Class Gonorrhea Antibiotic Developed Through Public-Private Partnership
November 1, 2023
A single dose of a novel oral antibiotic called zoliflodacin has been found to be as safe and effective as standard therapy for uncomplicated urogenital gonorrhea in an international Phase 3 non-inferiority clinical trial. Gonorrhea treatment options are increasingly limited due to antimicrobial resistance seen in Neisseria gonorrhoeae, the bacteria that cause gonococcal infection.

Study Reveals How Young Children’s Immune Systems Tame SARS-CoV-2
October 13, 2023
A study of infants and young children found those who acquired SARS-CoV-2 had a strong, sustained antibody response to the virus and high levels of inflammatory proteins in the nose but not in the blood. This immune response contrasts with that typically seen in adults with SARS-CoV-2 infection.
Clinical Trial to Test Immune Modulation Strategy for Hospitalized COVID-19 Patients Begins
September 22, 2023
A clinical trial has launched to test whether early intensive immune modulation for hospitalized COVID-19 patients with relatively mild illness is beneficial. The placebo-controlled study will enroll approximately 1,500 people at research sites around the world.
Clinical Trial of HIV Vaccine Begins in United States and South Africa
September 20, 2023
A trial of a preventive HIV vaccine candidate has begun enrollment in the United States and South Africa. The Phase 1 trial will evaluate a novel vaccine known as VIR-1388 for its safety and ability to induce an HIV-specific immune response in people.
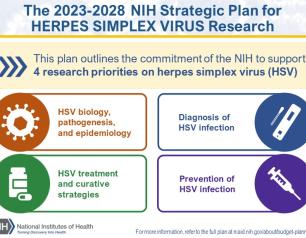
NIH Releases Strategic Plan for Research on Herpes Simplex Virus 1 and 2
September 19, 2023
In response to the persistent health challenges of herpes simplex virus 1 (HSV-1) and HSV-2, an NIH-wide HSV Working Group developed the plan, informed by feedback from more than 100 representatives of the research and advocacy communities and interested public stakeholders. The plan outlines an HSV research framework with four strategic priorities: improving fundamental knowledge of HSV biology, pathogenesis, and epidemiology; accelerating research to improve HSV diagnosis; improving strategies to treat HSV while seeking a curative therapeutic; and, advancing research to prevent HSV infection.
NIH Clinical Trial of Universal Flu Vaccine Candidate Begins
September 15, 2023
Enrollment in a Phase 1 trial of a new investigational universal influenza vaccine candidate has begun at the National Institutes of Health’s Clinical Center in Bethesda, Maryland. The trial is sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the NIH, and will evaluate the investigational vaccine for safety and its ability to elicit an immune response.
NIH Investigates Multidrug-Resistant Bacterium Emerging in Community Settings
September 6, 2023
New “hypervirulent” strains of the bacterium Klebsiella pneumoniae have emerged in healthy people in community settings, prompting a National Institutes of Health research group to investigate how the human immune system defends against infection.
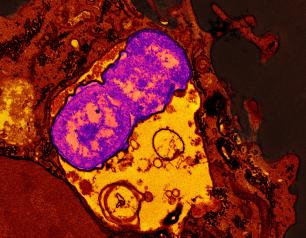
NIH Study Examines Connections Between Drinking Water Quality and Increased Lung Infections in People with Cystic Fibrosis
August 25, 2023
High levels of some minerals and metals in environmental water supplies may increase the risk of nontuberculous mycobacteria (NTM) pulmonary infections in people with cystic fibrosis. The study appearing in Environmental Epidemiology, found the presence of the metals molybdenum and vanadium along with sulfate—a collection of mineral salts—in the U.S. municipal water system was associated with an increased incidence of NTM pulmonary infections, the leading cause of drinking-water associated illnesses.
Severe COVID-19 May Lead to Long-Term Innate Immune System Changes
August 18, 2023
Severe COVID-19 may cause long-lasting alterations to the innate immune system, the first line of defense against pathogens, according to a small study funded by the National Institute of Allergy and Infectious Diseases.
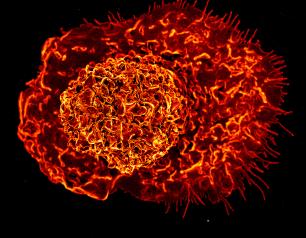
COVID-19 Vaccination and Boosting During Pregnancy Benefits Pregnant People and Newborns
August 11, 2023
Receiving a COVID-19 mRNA vaccine or booster during pregnancy can benefit pregnant people and their newborn infants, according to findings recently published in Vaccine. The paper describes results from the Multisite Observational Maternal and Infant Study for COVID-19 (MOMI-VAX), which was funded by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health.
NIH Selects Dr. Jeanne Marrazzo as Director of the National Institute of Allergy and Infectious Diseases
August 2, 2023
Lawrence A. Tabak, D.D.S., Ph.D., acting director for the National Institutes of Health (NIH), has named Jeanne M. Marrazzo, M.D., as director of NIH’s National Institute of Allergy and Infectious Diseases (NIAID).

Daily Statin Reduces Heart Disease Risk Among Adults Living with HIV
July 24, 2023
A National Institutes of Health-supported study found that statins, a class of cholesterol-lowering medications, may offset the high risk of cardiovascular disease in people living with HIV by more than a third, potentially preventing one in five major cardiovascular events or premature deaths in this population. People living with HIV can have a 50-100% increased risk for cardiovascular disease.
NIH Awards Will Fund Post-Treatment Lyme Disease Syndrome Research
July 21, 2023
Five projects awarded for research to better understand Post-treatment Lyme Disease Syndrome (PTLDS), which is a collection of symptoms, such as pain, fatigue, and difficulty thinking or “brain fog,” that linger following standard treatment for Lyme disease.

Investigational Three-Month TB Regimen Is Safe but Ineffective, NIH Study Finds
July 5, 2023
The first clinical trial of a three-month tuberculosis (TB) treatment regimen is closing enrollment because of a high rate of unfavorable outcomes with the investigational course of treatment. Advancing Clinical Therapeutics Globally for HIV/AIDS and Other Infections (ACTG) 5362, also known as the CLO-FAST trial, sought to evaluate the safety and efficacy of a three-month clofazimine- and high-dose rifapentine-containing regimen. An interim data analysis showed that participants taking the investigational regimen experienced ongoing or recurring TB at rates above thresholds set in the study protocol.
Screening Newborns for Deadly Immune Disease Saves Lives
June 20, 2023
Introducing widespread screening of newborns for a deadly disease called severe combined immunodeficiency, or SCID, followed by early treatment boosted the five-year survival rate of children with the disorder from 73% before the advent of screening to 87% since, researchers report. Among children whose disease was suspected because of newborn screening rather than illness or family history, 92.5% survived five years or more after treatment.
NIH Statement on HIV Vaccine Awareness Day 2023
May 18, 2023
Today marks the 26th observance of HIV Vaccine Awareness Day. The National Institutes of Health applauds the efforts of the collaborative global community of scientists, advocates, study participants, study staff, and funders enabling unprecedented levels of innovation and adaptation in the pursuit of a highly effective HIV vaccine.
First-in-Human Trial of Oral Drug to Remove Radioactive Contamination Begins
May 15, 2023
A first-in-human clinical trial of an experimental oral drug for removing radioactive contaminants from inside the body has begun. The trial is testing the safety, tolerability and processing in the body of escalating doses of the investigational drug product HOPO 14-1 in healthy adults. The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, is funding the Phase 1 trial, which is sponsored and conducted by SRI International of Menlo Park, California.

Clinical Trial of mRNA Universal Influenza Vaccine Candidate Begins
May 15, 2023
A clinical trial of an experimental universal influenza vaccine developed by researchers at the National Institute of Allergy and Infectious Diseases’ (NIAID) Vaccine Research Center (VRC), part of the National Institutes of Health, has begun enrolling volunteers at Duke University in Durham, North Carolina. This Phase 1 trial will test the experimental vaccine, known as H1ssF-3928 mRNA-LNP, for safety and its ability to induce an immune response.
NIH Celebrates FDA Approval of RSV Vaccine for People 60 Years of Age and Older
May 4, 2023
Food and Drug Administration announced the approval of the first respiratory syncytial virus (RSV) vaccine approved for use in the United States. The vaccine, Arexvy, is approved for the prevention of lower respiratory tract disease caused by RSV in individuals 60 years of age and older.
NIH Researchers Uncover New Details on Rare Immune Disease
May 3, 2023
In an 11-year study, researchers at the National Institutes of Health have further characterized idiopathic CD4 lymphocytopenia (ICL), a rare immune deficiency that leaves people vulnerable to infectious diseases, autoimmune diseases and cancers. Researchers observed that people with the most severe cases of ICL had the highest risk of acquiring or developing several of the diseases associated with this immune deficiency. This study, published in the New England Journal of Medicine, was led by Irini Sereti M.D., M.H.S. and Andrea Lisco, M.D., Ph.D.