NIAID scientists have found an association between widely used chemicals called diisocyanates and atopic dermatitis, a chronic inflammatory skin disease commonly known as eczema. The researchers also found through an experiment in mice that when bacteria that normally reside on skin are exposed to isocyanate, a component of diisocyanates, they adapt by stopping production of the oils that skin needs to stay healthy. These findings were recently published in the journal Science Advances.
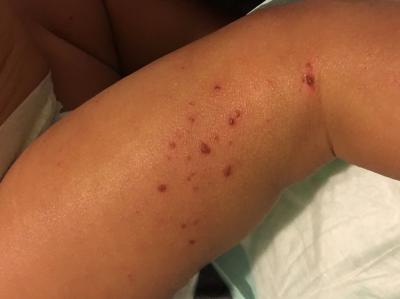
Eczema affects up to 20% of children and up to 10% of adults in high-income countries. People with eczema experience severe itching, skin redness, oozing from the skin, and scaly rashes, all of which can be painful. A particularly stressful aspect of eczema is its unpredictability, as symptoms can worsen from exposure to multiple substances or without any obvious trigger.
Prior to 1970, the prevalence of eczema in the United States was three to six times lower than it is today. While genetic risk factors are known to play a strong role in the incidence of eczema, the rapidly increasing prevalence of the disease indicates that environmental factors are also important. NIAID researchers led by Ian Myles, M.D., M.P.H., sought to identify environmental pollutants that may be contributing to this increase. Dr. Myles is chief of the Epithelial Research Unit in the NIAID Laboratory of Clinical Immunology and Microbiology.
The investigators compared a database that collates clinic visits for atopic dermatitis with two Environmental Protection Agency resources: the Toxics Release Inventory, which tracks the amounts of certain toxic chemicals that are released into the environment for each U.S. zip code; and the Risk-Screening Environmental Indicators model, which models the route of each chemical release through the environment and the potential human exposure that may result. The scientists identified emissions of diisocyanates as the strongest predictor of local eczema rates.
Diisocyanates have been used since the late 1940s to make polyurethane products, such as rigid and flexible foams, coatings, adhesives, sealants, and elastomers (rubbery materials). Exposure to these products has been shown to increase the risk of eczema, according to the NIAID researchers. The active portion of the diisocyanate molecule, the isocyanate side chain, is also a component of other known eczema triggers, such as wildfires, cigarette smoke, and automobile exhaust from catalytic converters, which became mandatory in the United States in 1975.
The investigators conducted experiments on the skin of mice to explore how isocyanates might contribute to eczema. Previous research demonstrated that exposing mouse skin to diisocyanates could directly induce eczema, yet the mechanisms were unclear. The current study found that when bacteria that live on healthy skin are exposed to isocyanate, they must adapt to survive. When they adapt, these bacteria shift their metabolism away from making the lipids, or oils, that skin needs to stay healthy. This finding suggests that eczema may be treatable by replacing the modified skin bacteria with healthy bacteria, according to the scientists.
Further research is needed to validate the association between environmental exposure to isocyanate or diisocyanates and eczema and to determine whether the causative mechanisms identified in mice are also found in people.
Dr. Myles and colleagues previously reported the results of a small, early-phase clinical trial that found applying healthy versions of a skin bacterium called Roseomonas mucosa to the skin of people with eczema led to a significant reduction in eczema symptoms that persisted even after the medication was stopped. A subsequent, larger, placebo-controlled clinical trial of R. mucosa strains found that the data failed to meet statistical significance for the primary endpoint of EASI-50 (the proportion of patients with at least a 50% improvement in atopic dermatitis disease severity as measured by the Eczema Area and Severity Index). However, the subgroup of study participants who completed the Phase 2 trial demonstrated sustained, modest, but statistically significant clinical improvements that differed by study site diisocyanate levels.
To facilitate their availability as a potentially beneficial probiotic, NIAID is making the R. mucosa strains available for commercial, non-therapeutic development.
References:
J Zeldin et al. Exposure to isocyanates predicts atopic dermatitis prevalence and disrupts therapeutic pathways in commensal bacteria. Science Advances DOI: 10.1126/sciadv.ade8898 (2023).
SM Langan et al. Atopic dermatitis. The Lancet DOI: 10.1016/S0140-6736(20)31286-1 (2020).
BC Martel et al. Translational animal models of atopic dermatitis for preclinical studies. Yale Journal of Biology and Medicine (2017).
IA Myles et al. Therapeutic responses to Roseomonas mucosa in atopic dermatitis may involve lipid-mediated TNF-related epithelial repair. Science Translational Medicine DOI: 10.1126/scitranslmed.aaz8631 (2020).