Are you involved in planning and implementing international clinical research? If so, we welcome you to join the more than 67,000 visitors from 167 countries from the past year who used NIAID’s ClinRegs website.
The online database serves as a central resource for country-specific clinical research requirements for regulatory and ethics approval, clinical trial lifecycle, sponsor responsibilities, informed consent, investigational products, and specimens. Information for 23 countries is now available.
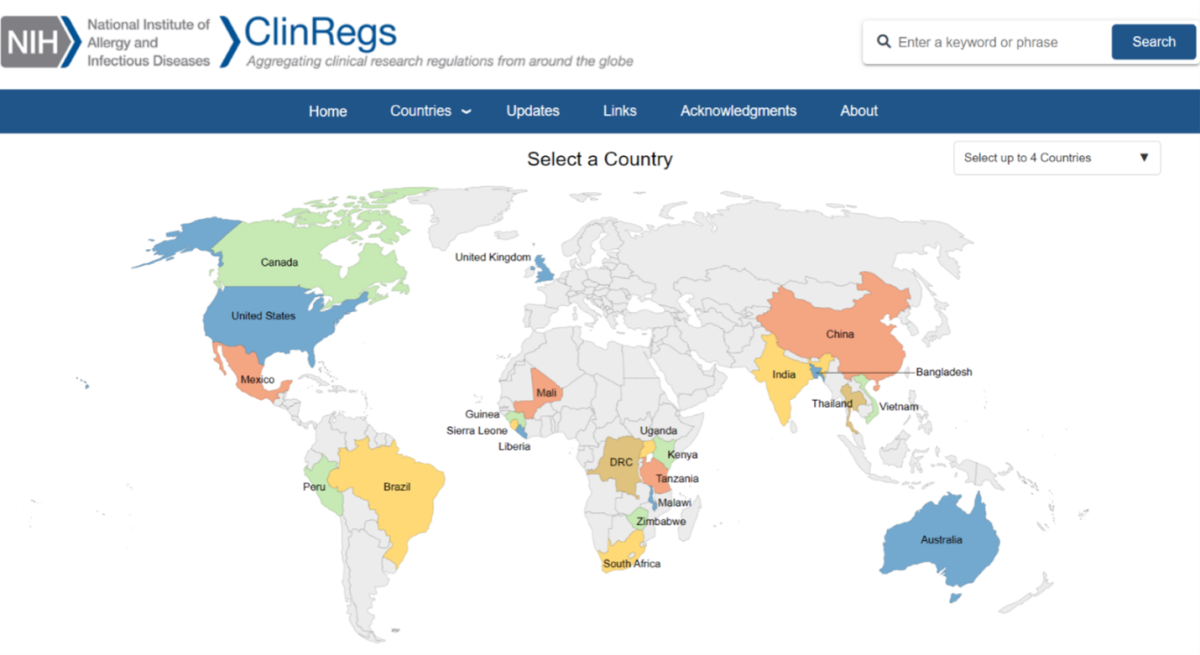
By serving as a one-stop shop for clinical research requirements, ClinRegs can save you time and effort. The site offers benefits such as:
- Plain language narrative descriptions of country-specific clinical research requirements
- Links to official regulatory and guidance documents
- English translations (where available)
- A country comparison feature
- Embedded alerts about recent changes to country requirements
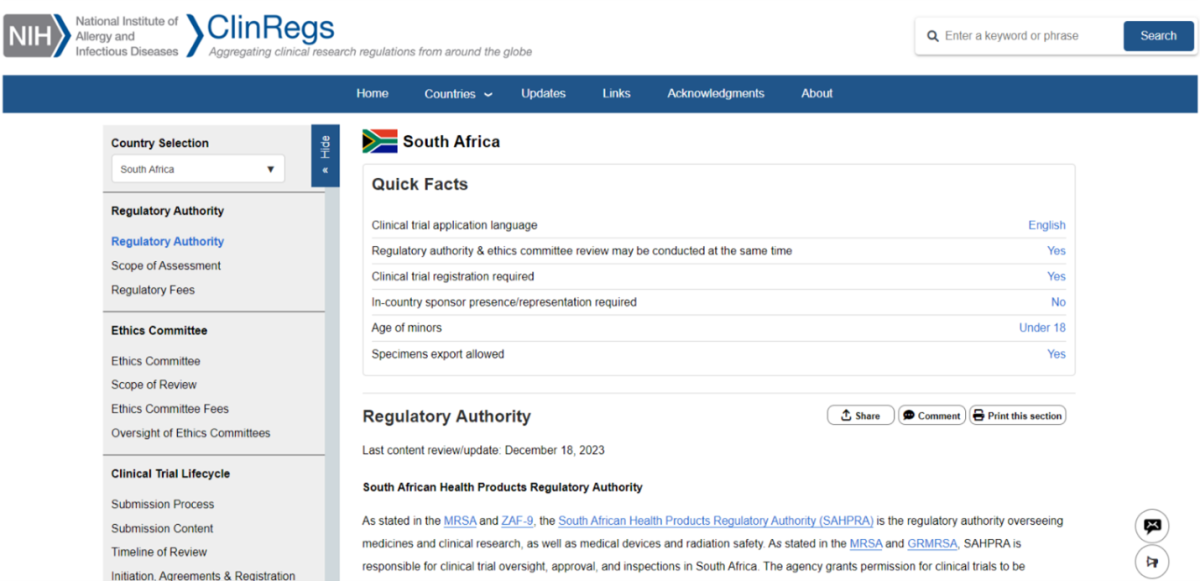
To ensure our content is current and accurate, NIAID’s ClinRegs team works closely with country subject matter experts to update the country profiles yearly. The group makes updates more frequently as needed to incorporate substantial, timely changes to country regulations or requirements, or to address broken website links.
Visitors to the site are encouraged to interact with the content and share their expertise by using the share and comment buttons found in each country profile section, or through the Contact Us link at the top of each page.
To get the latest updates on clinical research requirements, sign up for the General News and Announcements listserv, follow ClinRegs on X, or keep an eye out for ClinRegs posts on NIAID’s LinkedIn page.
If you have any questions or comments, email the team at NIAIDClinRegsSupport@mail.nih.gov.

