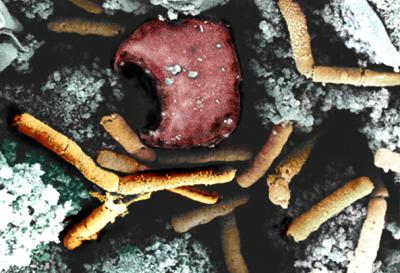
Color-enhanced scanning electron micrograph shows inhalational anthrax; featured are rod-shaped bacilli (yellow) and an erythrocyte (red).
Inhalation anthrax is the deadliest form of infection caused by the bacteria Bacillus anthracis. When a person breathes in anthrax spores, infection begins in the lungs and is carried to the chest lymph nodes. It then spreads throughout the body via the bloodstream, causing severe breathing problems and shock. Immediate treatment is crucial to survival. Without treatment, only about 10 to 15 percent of patients with inhalation anthrax survive.
New treatment options for inhalation anthrax are needed. NIAID, in coordination with the HHS Biomedical Advanced Research and Development Authority (BARDA), supported preclinical and clinical research to develop several monoclonal antibody-based therapeutics as anthrax antitoxins. One such product is Anthim, a human monoclonal antibody (mAb) produced by Elusys Therapeutics, Inc. In combination with antimicrobials, Anthim is intended to be used as an intravenous adjunct treatment for patients with inhalation anthrax and to help prevent disease after exposure to inhaled anthrax spores.
NIAID supported the preclinical and clinical development of Anthim through both grants and contracts several resources. Initially, funding was provided by BARDA and contract administration was performed at NIAID. NIAID then worked with Elusys to develop a scalable process and produce a large quantity of a stable formulation of Anthim. The mAb demonstrated efficacy in animal models and in small-scale, Phase 1 clinical trials, was shown to be safe for humans. NIAID then advanced the development of Anthim by supporting the manufacture of several pilot lots. Following the success of these efforts, BARDA awarded Elusys an advanced development contract to support final development stages.
In 2016, the Food and Drug Administration approved Anthim for the treatment of inhalational anthrax in combination with appropriate antibacterial drugs, and for the prevention of inhalational anthrax when alternative therapies are not available or not appropriate.

