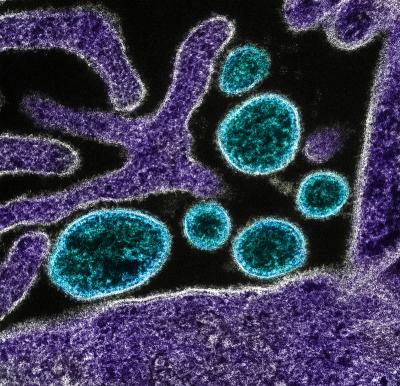
Colorized transmission electron micrograph of mature extracellular Nipah Virus particles (blue) near the periphery of an infected VERO cell (purple). Image captured and color-enhanced at the NIAID Integrated Research Facility in Fort Detrick, Maryland.
Hendra virus is an emerging virus that was first discovered in 1994 in Australian horses. Hendra occurs naturally in bats, which can transmit the virus to horses. All human cases of Hendra viruses have occurred following direct exposure to infected horses. Although Hendra infection is rare in people, 57 percent of individuals with confirmed Hendra infection have died, and some survivors have lasting neurological effects.
Nipah virus is a close relative of the Hendra virus and also causes fatal infections in animals and humans. Like Hendra, Nipah is carried by bats, which can transmit the virus to pigs and humans. Nipah has infected humans who have had close contact with infected pigs or bat secretions and can spread from person to person through close contact. There have been more than a dozen Nipah virus outbreaks to date, with most occurring in Bangladesh. Human case fatality rates have been between 40 and 100 percent. Due to the high mortality rate of Hendra and Nipah infection, and the sporadic and unpredictable nature of outbreaks, effective therapeutics and vaccines are needed.
NIAID-funded researchers at the Uniformed Services University of the Health Sciences (USU) and their collaborators at the National Cancer Institute discovered a potential antibody treatment for Nipah and Hendra virus. The researchers developed a human monoclonal antibody (mAb) known as m102.4 that targets the G glycoprotein of both viruses and found that the mAb effectively protected ferrets after exposure to Nipah or Hendra virus. The mAb was also effective in protecting nonhuman primates after exposure. This nonhuman primate model for Nipah and Hendra was developed with NIAID support by the United States Army Medical Research Institute for Infectious Diseases (USAMRIID), the NIAID Rocky Mountain Laboratories, and the University of Texas Medical Branch. NIAID preclinical services have been used to evaluate m102.4 in tissue culture, create a method to produce the mAb, and improve mAb formulation and stability.
After exposure to Hendra through infected horses, several people in Australia received the therapeutic (under emergency use authorization) with no adverse effects. The Australian government is currently manufacturing and stockpiling mAb m102.4 to use during future outbreaks, and has recently evaluated it in a Phase I clinical trial. The results of this trial showed that m102.4 was safe and well-tolerated, supporting the use of this mAb in humans following high-risk exposure to Nipah or Hendra viruses.
NIAID’s Partnerships program has also supported the development of a Hendra vaccine for horses. Vaccinating horses against Hendra will help to prevent them from becoming infected with the deadly virus and is expected to prevent horse-to-human virus transmission. NIAID-funded researchers at USU created the vaccine using one of the Hendra virus proteins (sG) that is essential for infection. The vaccine was evaluated using the same nonhuman primate model supported by NIAID and used to test the mAb. After further testing in animal models, USU researchers partnered with Pfizer Animal Health and Australia's Commonwealth Scientific and Industrial Research Organization to produce the vaccine for horses. In November 2012, Equivac® HeV became the world's first commercially available Hendra vaccine for horses and has been shown to protect experimentally infected cats, ferrets, and monkeys against Nipah virus.
Ongoing studies funded by the NIAID Centers of Excellence for Translational Research involve extending the therapeutic window of m102.4, isolation of pan-henipavirus mAbs and antibody cocktails which neutralize both Nipah and Hendra viruses, and the development of a vaccine for use in humans. NIAID-funded researchers have demonstrated that the vaccine protects monkeys from Nipah and Hendra virus infection, an important first step for future use in humans.

