Criteria May Help Guide Treatment of Dermatitis
March 14, 2025
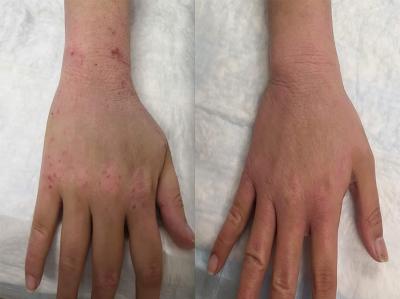
Hand and wrist of a participant in the pilot study before (left) and after (right) treatment of topical steroid withdrawal symptoms with berberine, a mitochondrial complex I-blocking drug.
Researchers at the National Institutes of Health (NIH) have determined that dermatitis resulting from topical steroid withdrawal (TSW) is distinct from eczema and is caused by an excess of an essential chemical compound in the body. Scientists from NIH’s National Institute of Allergy and Infectious Diseases (NIAID) identified treatments that could be studied in clinical trials for the condition based on their potential to lower levels of the chemical compound—called nicotinamide adenine dinucleotide (NAD+), a form of vitamin B3. The findings were published today in the Journal of Investigative Dermatology.
Dermatitis is characterized by inflammation, itching, or burning sensations on the skin, and can result from various conditions including TSW and eczema. Eczema, also known as atopic dermatitis, is a common cause of dermatitis and affects 10 to 30% of children and 2 to 10% of adults each year in the United States. Topical steroids—specifically glucocorticoids or topical corticosteroids—have long been used as a first-line treatment for dermatitis caused by eczema because the drugs are safe, effective, easy to apply, and considered well-tolerated.
Some people experience dermatitis after using topical steroids for prolonged periods of time and then stopping—a condition called TSW. Diagnosing and treating this condition is difficult because TSW is not well understood. Symptoms include skin redness, burning sensations, skin heat (thermal dysregulation), itching and peeling, which can even occur on parts of the body where topical steroids were not applied. As TSW and eczema have similar symptoms, it has been difficult to distinguish the two disorders.
To better understand TSW, a team led by scientists in NIAID’s Laboratory of Clinical Immunology and Microbiology evaluated a previous survey that included 1,889 adults with symptoms similar to eczema. By dividing the participants into those with self-reported TSW and those without, the researchers identified characteristics unique to TSW. The researchers then conducted a pilot study including 16 people with symptoms consistent with TSW, 10 people with eczema but no symptoms of TSW, and 11 people without skin disease. They found that people with TSW symptoms had elevated levels of NAD+ in their blood serum and skin, while NAD+ levels were within a typical range in people without TSW symptoms.
The researchers subsequently used cultured skin cells and a mouse model to mimic TSW conditions. They found that NAD+ was produced in response to topical steroids and caused inflammation. The models suggested that administration of a drug that blocked the formation of NAD+—called a mitochondrial complex I blockade—would improve TSW symptoms. In a pilot study to further assess this treatment strategy, the researchers evaluated subjective responses among study participants who used the mitochondrial complex I-blocking drugs metformin, berberine, or both. After three to five months of use, most participants reported improvement in TSW symptoms.
The scientists provisionally established criteria that can be used by health care providers to identify TSW in people. People who have stopped topical steroid treatment and meet the criteria may be diagnosed by practitioners as having TSW. The researchers suggest that patients identified as having TSW could be treated using the proposed mitochondrial complex I-blocking drugs.
The results of this study may help practitioners identify TSW in patients and work towards developing safe and effective treatments. According to the researchers, more research is needed to determine whether all patients with TSW have an excess of NAD+, or if there are other features that define TSW. Additionally, the diagnostic criteria will help health care providers and researchers to better understand the prevalence of TSW and evaluate the effects of using topical steroids.
ARTICLE:
N Shobnam, G Ratley, S Saksena et al. Topical Steroid Withdrawal is a Targetable Excess of Mitochondrial NAD+. Journal of Investigative Dermatology 10.1016/j.jid.2024.11.026 (2025).
WHO:
Ian Myles, M.D. M.P.H., Principal Investigator, Epithelial Therapeutics Unit in NIAID’s Laboratory of Clinical Immunology and Microbiology is available to discuss this research.
Contact
Submit a Media Request
Contact the NIAID News & Science Writing Branch.

