NIAID Research
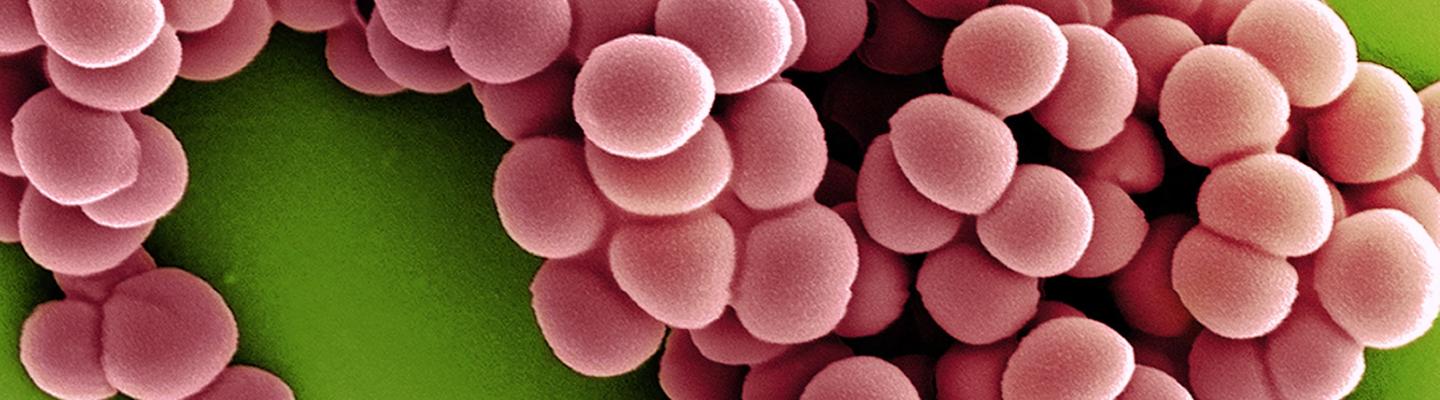
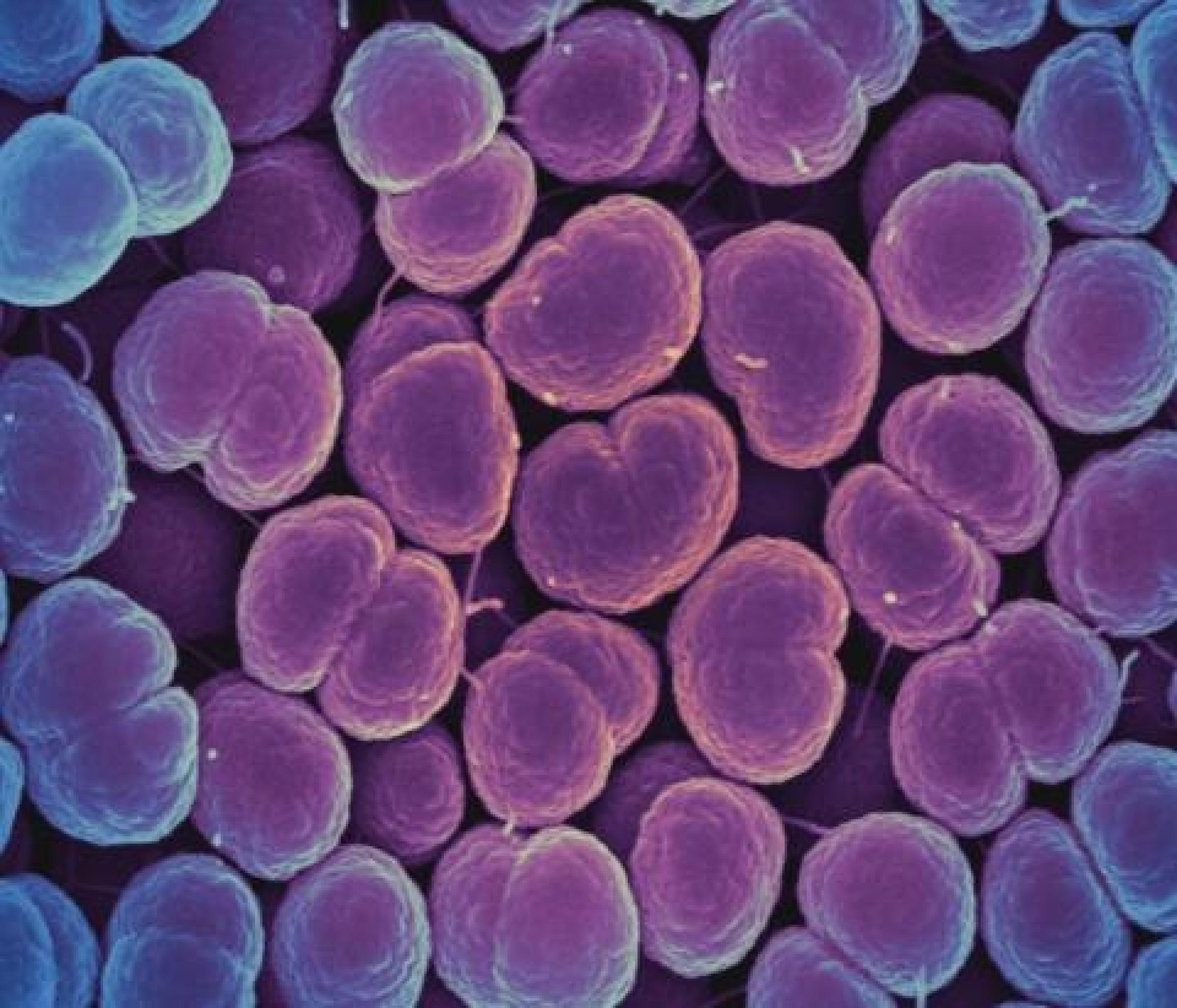
Without appropriate diagnostic tests, it can be difficult to know what type of pathogen is causing an infection. Viral infections can be mistaken for bacterial infections and improperly treated with antibiotics, while bacterial infections can be treated with the wrong antibiotic, causing prolonged disease. Furthermore, standard diagnostic tests can take two to three days or longer to yield a result. The process involves culturing the infecting organism to identify the species and performing tests to see which types of antibiotics work against that particular strain. Reliable, rapid tests are urgently needed to avoid delays in appropriate treatment.
NIAID is supporting the development of rapid, multiplexed diagnostics platforms and biomarkers. This includes tests that can identify if a pathogen will be susceptible to a particular antibiotic, tests that can distinguish colonization from infection and tests that do not require culturing bacteria.
NIAID scientists are studying the mechanisms of antimicrobial resistance and novel approaches for detecting resistance based on large-scale studies of proteins and other biomarkers. In addition, NIAID’s Antibacterial Resistance Leadership Group (ARLG) is testing a biomarker that could distinguish bacterial viral and bacterial infections to determine if antibiotics are necessary. The ARLG also conducts studies on improving the utility of current diagnostics. An ARLG study showed that a test previously approved by the FDA to detect gonorrhea and chlamydia in urine and reproductive samples could also be used to accurately detect gonorrhea and chlamydia in throat and rectum samples.
Antimicrobial Resistance Diagnostic Challenge
NIH and BARDA are supporting a federal prize competition seeking to identify innovative and rapid point-of-need diagnostic tests to help inform appropriate antibiotic treatment and facilitate antimicrobial stewardship efforts. Read more about the challenge.