Vaccine Development Unit
Patrick E. Duffy, M.D.
Chief, Laboratory of Malaria Immunology and Vaccinology
Chief, Vaccine Development Unit
Chief, Pathogenesis and Immunity Section
Major Areas of Research
- Process development of vaccine candidates for commercial viability, including molecular biology, fermentation/purification, conjugation, formulation, and quality control
- Preclinical evaluation of vaccine candidates
- Clinical trials, both U.S. and international
- Immunologic, molecular, and entomologic assay development
Program Description
LMIV Vaccine Development Unit develops and performs clinical evaluation of prototype malaria vaccines. Each candidate vaccine must undergo a rigorous development process that requires input from many highly skilled scientists with specific areas of expertise. These include creation of expression systems, fermentation optimization, scale-up of purification technology, clinical good manufacturing practices (cGMP) production, formulation, quality control, preclinical testing, and clinical trials.
This product-oriented research differs from investigator-initiated research conducted in most laboratories. The need for multiple, highly technical inputs makes it impractical to have a single person knowledgeable in all aspects of a specific product.
For these reasons, the unit has adopted an organizational structure used by most biotechnology companies to conduct this development process. This model incorporates best practices from both public and private sectors to rapidly advance vaccine products into Phase II clinical trials. Our organizational approach is coupled with creative management practices, allowing us to operate and fund the program within the framework of the federal government. In addition, the LMIV collaborates with numerous private and public research organizations across the globe; see a comprehensive list of collaborating organizations and the research topics conducted with each.
The Vaccine Development Unit has focused research to eradicate malaria through development of a vaccine to interrupt malaria transmission (VIMT), including transmission-blocking components and pre-erythrocytic components. Another goal of the Vaccine Development Unit is to design and develop vaccines specifically to protect pregnant women and their infants.
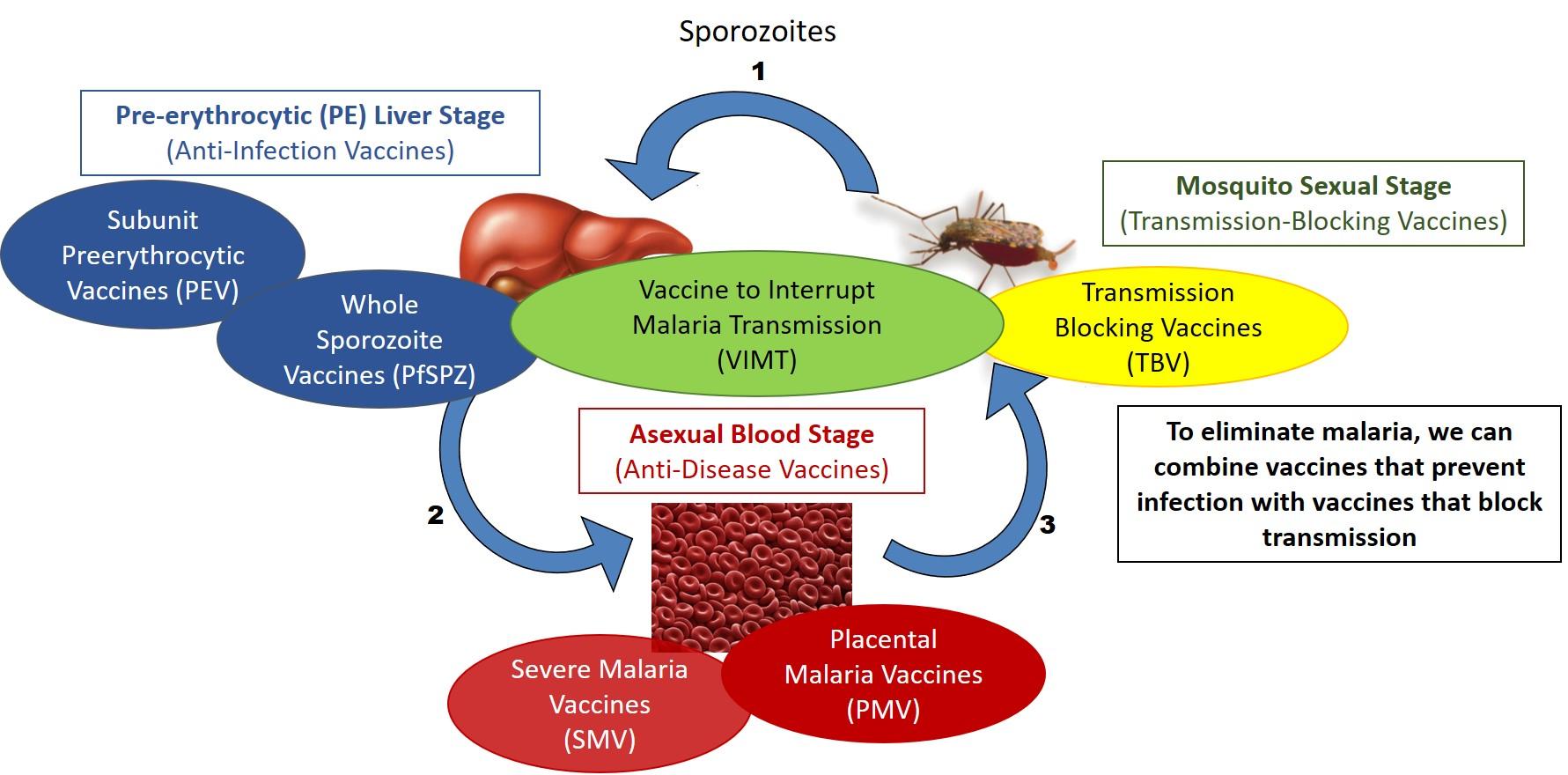
Malaria vaccines target different stages of the parasite life cycle.
Transmission-Blocking Vaccines
The objective of this program is to develop a transmission-blocking vaccine (TBV) that would eliminate malaria transmission in low-transmission areas and reduce disease burden in moderate- and high-transmission areas. TBVs are unlike other malaria vaccine candidates since they do not protect vaccines from infection or disease but are designed to prevent infection of mosquitoes and halt further transmission within a community. The LMIV pioneers TBV research while also developing pre-erythrocytic vaccines (see below), in an effort to combine both components to form what is known as a Vaccine to Interrupt Malaria Transmission (VIMT), which would prevent both disease and transmission in a community.
The LMIV’s leading TBV candidate, named Pfs230D1, induces antibodies that target sexual stages of malaria parasites which develop in the mosquito. In a phase 1 clinical trial, Pfs230D1 was safe and induced transmission-reducing activity in volunteers from both the US and malaria-endemic regions of Mali. Pfs230D1 is currently under phase 2 clinical evaluation of safety and functional activity within a community in Mali. See the recent news release announcing our planned clinical trials of TBVs throughout West Africa, in collaboration with partners from the Netherlands, Denmark, Mali, Burkina Faso, Liberia, and Guinea.
Pre-Erythrocytic Stage Vaccines
Pre-erythrocytic stage vaccines are designed to induce immunity against the sporozoites injected by mosquitoes or the ensuing liver stage parasites, and thereby prevent the early stages of malaria infection from developing into blood-stage infection and disease. An effective pre-erythrocytic vaccine could thus prevent clinical disease and also disrupt malaria transmission. Even a partially effective vaccine would significantly lower initial parasite burden, which would substantially reduce the frequency and severity of clinical malaria.
The current leading pre-erythrocytic stage vaccine is RTS,S (Mosquirix, developed by GlaxoSmithKline), which has advanced through a phase 3 clinical trial in over 15,000 infants and young children, the largest malaria vaccine trial in Africa to date. The trial demonstrated the vaccine was safe and prevented ~40% of malaria cases and ~30% of severe malaria over a four-year period (see RTS,S fact sheet ). This progress represents a historic milestone, and after favorable review by The European Medicines Agency and the World Health Organization, RTS,S has now entered a pilot implementation program in Malawi, Ghana, and Kenya. In an effort to further improve the efficacy of this vaccine, the LMIV has identified other pre-erythrocytic antigens and demonstrated that these can enhance protection conferred by circumsporozoite protein, the primary antigen used in RTS,S.
The LMIV has also worked in close partnership with Sanaria, Inc. to understand sterilizing immunity induced by another pre-erythrocytic stage vaccine called PfSPZ Vaccine. PfSPZ Vaccine is comprised of irradiated whole sporozoites, and has been shown to induce protective efficacy greater than that reported for RTS,S in adults. In addition, the LMIV is currently developing with Sanaria an alternate approach called PfSPZ chemoprophylaxis vaccination, or PfSPZ-CVac, in which live sporozoites are injected while under protective cover of antimalarial drugs. Initial phase 1 studies of PfSPZ-CVac demonstrated this approach was safe and achieved unprecedented high levels of sterile immunity in malaria-naïve US volunteers; LMIV, Sanaria, and Mali partners are now conducting clinical field trials of PfSPZ-CVac in malaria-endemic areas of Mali.
Placental Malaria Vaccines
Pregnant women and their infants bear the greatest risk of severe outcomes caused by malaria, as pregnant women living in an endemic area become more susceptible to infection despite pre-existing immunity acquired from childhood. This is because malaria parasites can specifically attack the placenta and cause many adverse consequences such as maternal and infant anemia, low birth weight, and even death. We have observed that first-time pregnant mothers are at the greatest risk, and women acquire immunity against placental malaria over successive pregnancies. This suggests that a vaccine specifically to prevent placental malaria is achievable, if it can effectively induce those antibodies seen in protected women.
Placental malaria results when malaria-infected blood cells sequester in the placenta, binding to the placental receptor chondroitin sulfate A (CSA). The parasite protein responsible for this binding is called VAR2CSA, which is currently the primary target of placental malaria vaccines. However, the size and complexity of VAR2CSA are a challenge to large-scale vaccine production, thus, studies have mainly focused on defining smaller regions (or domains) of the protein. LMIV and others have shown that antibodies to some recombinant VAR2CSA domains (or domain combinations) may induce antibodies that inhibit parasite binding to CSA on placental cells, however none of these have induced the broadly neutralizing antibodies that are naturally acquired by women over successive pregnancies. Another challenge is that placental malaria vaccine protection observed in rodent animal models has not translated to efficacy in humans.
The LMIV has recently developed a nonhuman primate model in Aotus monkeys that recapitulates the major features of PM seen in pregnant women. Since full-length VAR2CSA immunogens may induce superior protection to recombinant domains, the LMIV also recently achieved viable expression of full-length VAR2CSA and is planning studies to test its safety and functional activity in the new Aotus model.
In addition, in collaboration with Sanaria, Inc. and the Malaria Research and Training Center in Bamako, Mali, with grant support from the Grand Challenges Saving Lives at Birth program, LMIV is pioneering the use of malaria vaccines to protect pregnant women from malaria. The funded project seeks to first test the safety and efficacy Sanaria’s PfSPZ Vaccine to specifically protect at-risk women of child-bearing potential against malaria in pregnancy, which will then culminate in a trial in at-risk pregnant women.
Biography
Education
M.D., Duke University, Durham, NC
Dr. Duffy completed medical school at Duke University, internal medicine training at Walter Reed Army Medical Center, and postdoctoral research training in molecular vaccine development at NIAID. He now leads research teams that focus on malaria pathogenesis, immunity, and vaccine development. He has extensive experience leading human observational and interventional studies of malaria, as well as mentorship of young scientists in US and Africa. Together with his LMIV colleague Dr. Michal Fried, he conducted seminal studies that revealed the mechanistic basis for placental malaria infection and protective immunity. As the Chief of the Laboratory of Malaria Immunology and Vaccinology (LMIV) at NIH, he is responsible for the intramural NIAID program to develop and test malaria vaccines in clinical trials. Under his leadership, LMIV has developed the first malaria transmission-blocking vaccines to enter field trials, has partnered with the local biotech Sanaria to conduct the first field efficacy trials of whole organism malaria vaccines, and is now pioneering a path in partnership with Mali colleagues to test the most promising malaria vaccines in pregnant women. Before taking the position at LMIV in Nov 2009, he served as Malaria Program Director at Seattle Biomedical Research Institute (now part of Seattle Children’s Research Institute), and as Affiliate Professor of Pathobiology and of Global Health at the University of Washington. In Seattle, at NIH, and in Mali, he and his partners established facilities for controlled human malaria infection (CHMI) studies with P. falciparum or P. vivax, and while at NIH, he has pioneered the use of mosquito feeding studies on human subjects as an endpoint for field trials of vaccines that will be used for malaria elimination.
Selected Publications
Healy SA, Murphy SC, Hume JCC, Shelton L, Kuntz S, Van Voorhis WC, Moodie Z, Metch B, Wang R, Silver-Brace T, Fishbaugher M, Kennedy M, Finney OC, Chaturvedi R, Hobbs CV, Warner-Lubin M, Talley AK, Wong-Madden S, Stuart K, Wald A, Kappe SH, Kublin JG, Duffy PE. Chemoprophylaxis vaccination: Phase 1 study to explore stage-specific immunity to Plasmodium falciparum in U.S. adults [published online ahead of print, 2019 Oct 17]. Clin Infect Dis. 2019;ciz1010.
Healy SA, Fried M, Richie T, Bok K, Little M, August A, Riley L, Swamy GK, Wylie BJ, Menendez C, Muehlenbachs A, Doumbo O, Greenwood B, Billingsley PF, Hoffman SL, Duffy PE. Malaria vaccine trials in pregnant women: An imperative without precedent. Vaccine. 2019 Feb 4;37(6):763-770.
Sagara I, Healy SA, Assadou MH, Gabriel EE, Kone M, Sissoko K, Tembine I, Guindo MA, Doucoure M, Niare K, Dolo A, Rausch KM, Narum DL, Jones DL, Macdonald NJ, Zhu D, Mohan R, Muratova O, Baber I, Coulibaly MB, Fay MP, Anderson C, Wu Y, Traore SK, Doumbo OK, Duffy PE. Safety and immunogenicity of Pfs25H-EPA/Alhydrogel, a transmission-blocking vaccine against Plasmodium falciparum: a randomised, double-blind, comparator-controlled, dose-escalation study in healthy Malian adults. Lancet Infect Dis. 2018;18(9):969‐982.
Sissoko MS, Healy SA, Katile A, Omaswa F, Zaidi I, Gabriel EE, Kamate B, Samake Y, Guindo MA, Dolo A, Niangaly A, Niaré K, Zeguime A, Sissoko K, Diallo H, Thera I, Ding K, Fay MP, O'Connell EM, Nutman TB, Wong-Madden S, Murshedkar T, Ruben AJ, Li M, Abebe Y, Manoj A, Gunasekera A, Chakravarty S, Sim BKL, Billingsley PF, James ER, Walther M, Richie TL, Hoffman SL, Doumbo O, Duffy PE. Safety and efficacy of PfSPZ Vaccine against Plasmodium falciparum via direct venous inoculation in healthy malaria-exposed adults in Mali: a randomised, double-blind phase 1 trial. Lancet Infect Dis. 2017;17(5):498‐509.
Zaidi I, Diallo H, Conteh S, Robbins Y, Kolasny J, Orr-Gonzalez S, Carter D, Butler B, Lambert L, Brickley E, Morrison R, Sissoko M, Healy SA, Sim BKL, Doumbo OK, Hoffman SL, Duffy PE. γδ T Cells Are Required for the Induction of Sterile Immunity during Irradiated Sporozoite Vaccinations. J Immunol. 2017;199(11):3781-3788.
Research Group
LMIV Vaccine Development Unit develops and performs clinical evaluation of prototype malaria vaccines. The VDU focuses on research to eradicate malaria through development of a vaccine to interrupt malaria transmission, including transmission-blocking components and pre-erythrocytic components. The VDU also designs and develops vaccines specifically to protect pregnant women and their infants.

