NIAID biodefense research runs the entire spectrum from basic research to the development of new diagnostics, drugs, and vaccines. The research program includes target identification, preclinical development, and clinical evaluation of experimental products. Research resources, such as new laboratories and genomics information, are also essential. The Institute's initiatives involve NIAID scientists, academia, and industry.
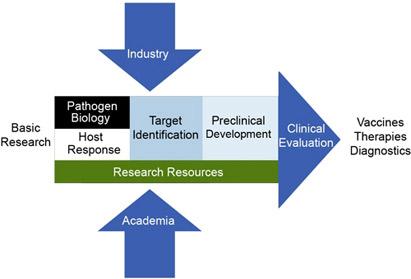
The inputs and stages of NIAID's biodefense research from basic research to the development of new diagnostics, drugs, and vaccines.
Funding initiatives that engage both academia and industry to put this plan into action fall into the following categories.
Basic Research
Basic research includes pathogen biology—research into the basic microbiology and pathogenesis of emerging infectious diseases and the microorganisms and toxins that may be used as agents of bioterrorism. Related activities include microbial genomic sequencing and proteomics.
Research also focuses on host response to these organisms and toxins. To develop potent, safe, and effective vaccines; accurate diagnostics; and immunotherapeutics, it is critical to improve our understanding of the complex parameters of innate and adaptive immunity. Because most potential bioterror agents would infect via the respiratory or oral routes, emphasis is also placed on studies of mucosal immunity at these sites. Crosscutting, multidisciplinary research will facilitate translation of this basic knowledge into vaccines, therapies, and diagnostic methods focused on bioterrorist agents. In addition, new discoveries of immunologic principles or applications will help ensure a robust pipeline of improved or novel products for biodefense, emerging infectious diseases, and many other diseases.
Target Identification
Basic research provides the foundation for identifying potential targets for new diagnostics, vaccines, and immunotherapies.
Preclinical Development
Preclinical development includes candidate product refinement testing that precedes human studies.
Clinical Evaluation
NIAID supports clinical evaluation of therapies, vaccines, and diagnostics for potential agents of bioterrorism and other emerging diseases through a number of initiatives and programs.
Research Resources
Resources include several initiatives to build research capacity and infrastructure. These initiatives also provide an array of reagent and other resources to facilitate the testing and development of promising candidates into effective products.

