The emergence of multidrug-resistant tuberculosis (MDR TB) and, more recently, extensively drug-resistant (XDR) TB has intensified the need for new TB drugs. Helping discover and develop those drugs is a top NIAID priority. The Institute supports research to elucidate the mechanisms of drug resistance, identify new TB drug targets and candidate drugs, and evaluate novel TB drugs and optimal drug combinations in preclinical and clinical studies.
Important advances in TB treatment research include
- Development of promising new drug candidates, some of which are currently being tested in human clinical trials
- Evaluation of shorter treatment regimens to make it easier for people to complete drug therapy
- Inclusion of antibiotics that are already available for treatment of other infections and have been shown to act on Mycobacterium tuberculosis may make therapy more potent and easier to tolerate
Read more about antibiotic-resistant Mycobacterium tuberculosis (TB).
Scientific Advances
NIH Clinical Trial of Tuberculous Meningitis Drug Regimen Begins
December 7, 2023A trial of a new drug regimen to treat tuberculous meningitis (TBM) has started enrolling adults and adolescents in several countries where tuberculosis (TB) is prevalent. The trial will include 330 participants aged 15 years and older who have or are likely to have TBM based on signs and symptoms, including people living with and without HIV. Because pregnant women are eligible to enroll in this…
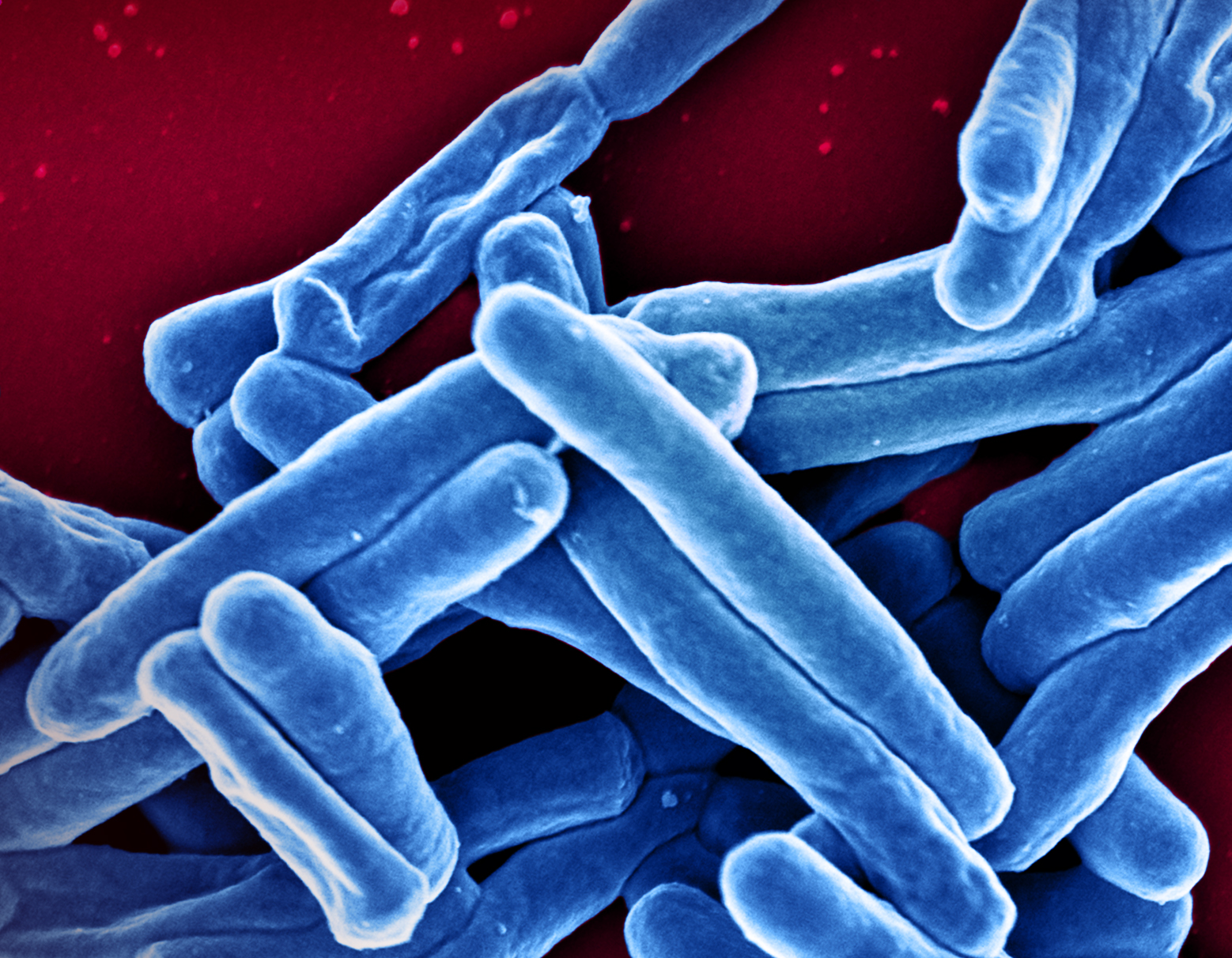
Investigational Three-Month TB Regimen Is Safe but Ineffective, NIH Study Finds
July 5, 2023The first clinical trial of a three-month tuberculosis (TB) treatment regimen is closing enrollment because of a high rate of unfavorable outcomes with the investigational course of treatment. Advancing Clinical Therapeutics Globally for HIV/AIDS and Other Infections (ACTG) 5362, also known as the CLO-FAST trial, sought to evaluate the safety and efficacy of a three-month clofazimine- and high…
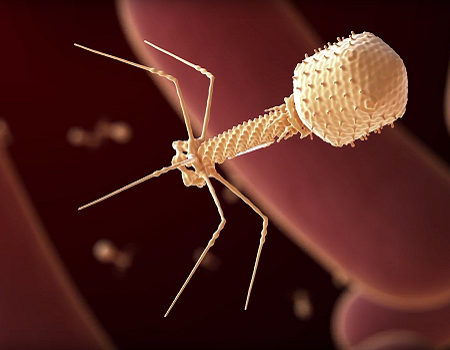
NIH Awards Grants to Support Bacteriophage Therapy Research
March 11, 2021NIAID has awarded $2.5 million in grants to 12 institutes around the world to support research on bacteriophage therapy.
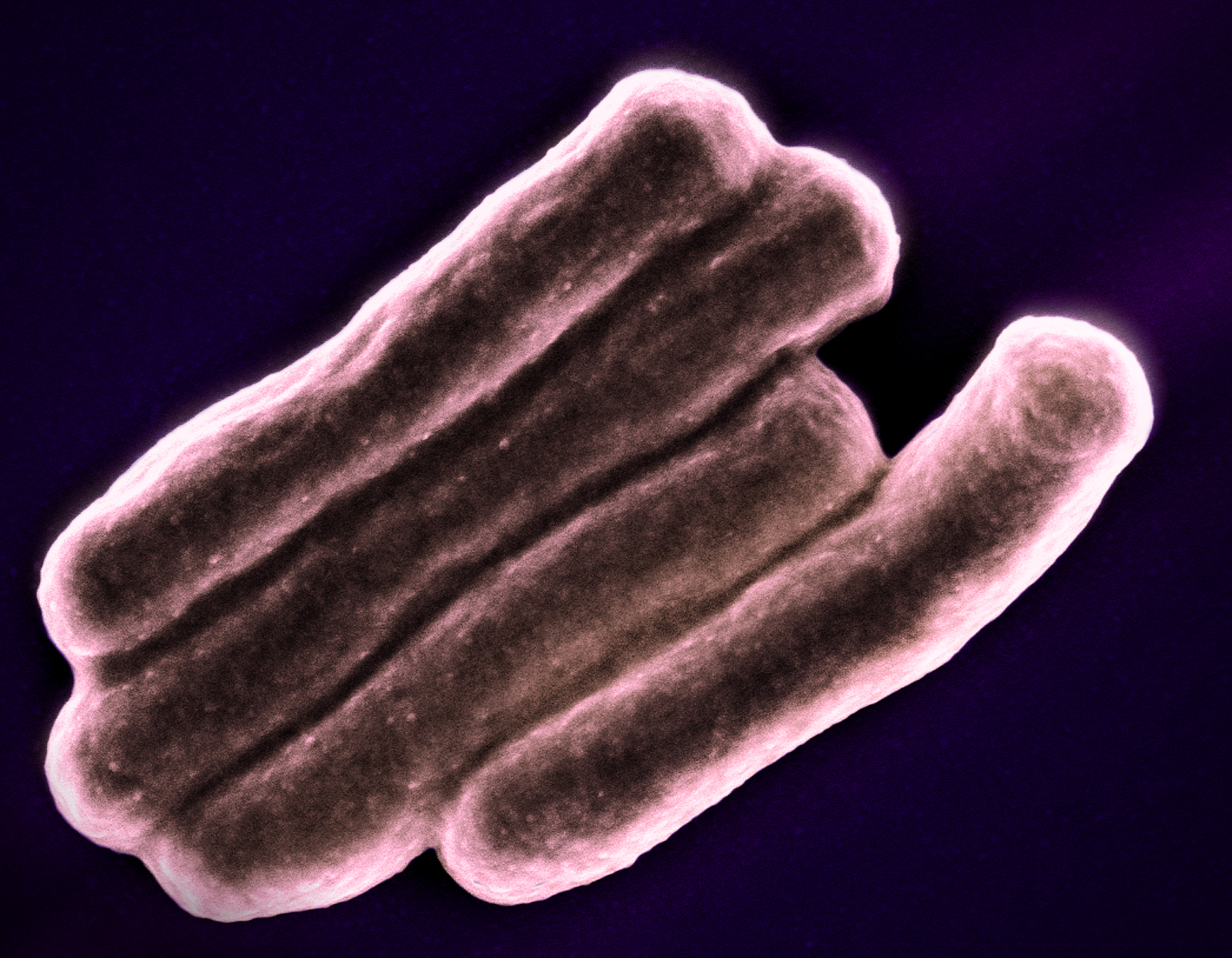
Landmark TB Trial Identifies Shorter-Course Treatment Regimen
October 21, 2020Results from a CDC and NIAID-sponsored clinical trial identify shorter-course treatment regimen for tuberculosis (TB).


