Type 1 Diabetes (T1D) is a life-threatening condition that occurs when the immune system’s T cells destroy insulin-producing beta cells in the pancreas. To survive, people with T1D must take insulin for the rest of their lives. Insulin is used to control blood sugar levels and reduce the risk of severe complications.
Stages of Type 1 Diabetes
T1D progresses in three distinct stages leading up to the clinical diagnosis of disease.
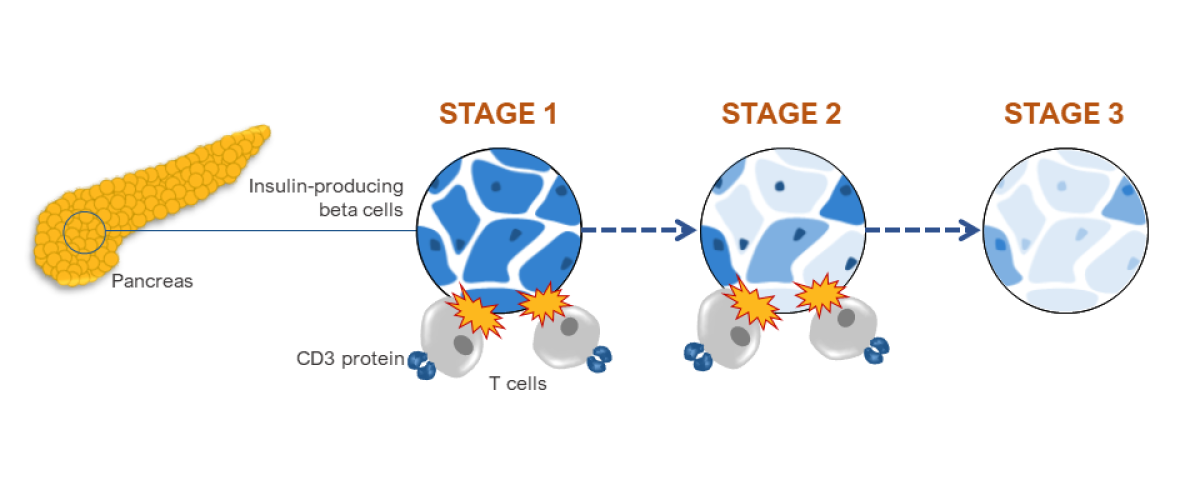
Stage 1: T cells begin to attack insulin-producing beta cells in the pancreas.
Stage 2: Some beta cells are destroyed, leading to abnormal blood sugar. T cells continue to attack remaining beta cells.
Stage 3: Nearly all beta cells are destroyed, leading to T1D symptoms.
Journey to T1D Treatment
Since the 1970s discovery that the immune system causes T1D, scientists have worked to develop medications that prevent the immune attack.
1970s
NIH-funded and private sector scientists discovered the antibody OKT3 binds the CD3 protein on T cells.
1980s
NIH-funded investigators showed that mice treated with an OKT3-like antibody prevented T1D. However, OKT3 caused unwanted side effects in humans.
1990s
Researchers discovered how OKT3 caused unwanted side effects and designed a safer antibody.
2000s
NIH-funded clinical trials evaluated the modified, safer antibody, which was named teplizumab.
2010s
NIH-funded and industry clinical trials evaluated the safety and efficacy of teplizumab in children in Stage 2 T1D.
2020s
FDA approved teplizumab in November 2022 to delay progression of T1D from Stage 2 to Stage 3.
How Teplizumab Works
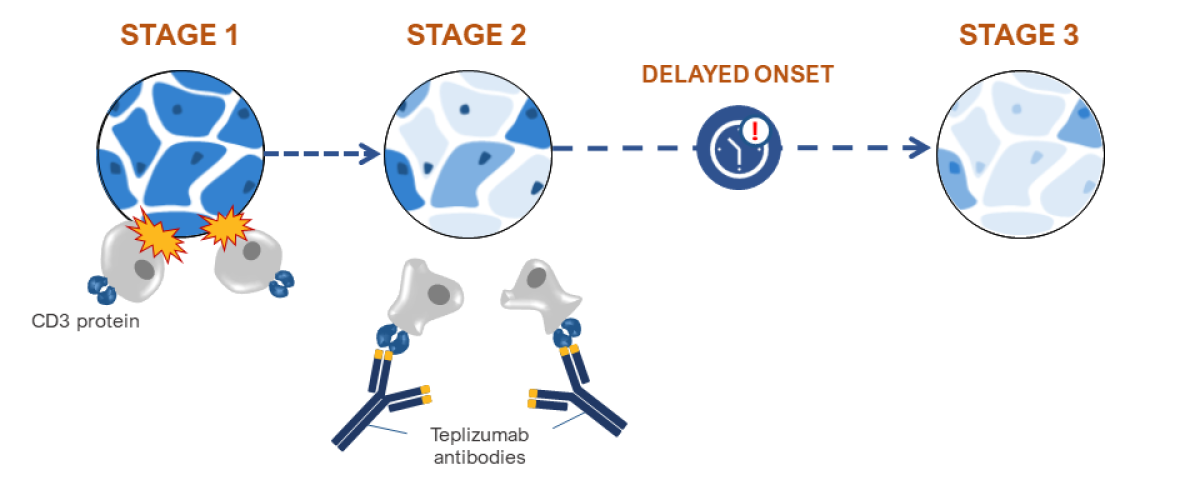
Stage 1: T cells begin to attack insulin-producing beta cells in the pancreas.
Stage 2: Some beta cells are destroyed, leading to abnormal blood sugar. Teplizumab now binds to CD3 on T cells, slowing their attack.
Delayed onset: Teplizumab slows T cell destruction of beta cells, delaying onset of Stage 3 T1D.
Stage 3: Nearly all beta cells are destroyed, leading to T1D symptoms.

