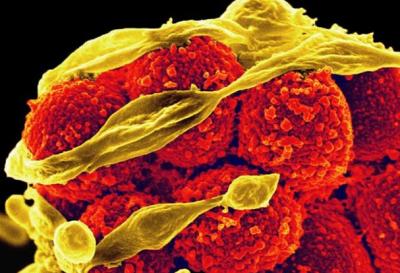
Colorized SEM of MRSA
The development of rapid, point-of-care diagnostics that can specifically identify the bacteria, fungi or virus causing an infection is an important step in combatting antibiotic resistance and is a priority for NIAID. Currently, broad spectrum antibiotics, which target a wide range of bacteria, are often prescribed when a diagnosis cannot be made prior to starting treatment. Diagnostics that identify the cause of an infection, as well as the susceptibility of that pathogen to specific antibiotics, can inform appropriate treatment, promote antibiotic stewardship, and ultimately, help fight antibiotic resistance.
NIAID has supported and continues to support a number of diagnostics to combat antibiotic resistance, including multiplex platforms such as the FilmArray Blood Culture Identification Panel by BioFire Diagnostics LLC, an affiliate of Biomérieux. Through small business grants and partnerships grants, NIAID supported the development of this polymerase chain reaction (PCR)-based system, which has been cleared by the Food and Drug Administration, to simultaneously detect several pathogens in patient samples in around one hour. This panel tests for 24 Gram-positive bacteria, Gram-negative bacteria, and yeast microbes that cause bloodstream infections (sepsis). The future assay under development will simultaneously tests for 24 pathogens; Gram-positive bacteria, Gram-negative bacteria, and yeast microbes that cause bloodstream infections (sepsis) within 2 hours of sample draw.
NIAID has also provided support for other novel approaches to diagnostics, including using digital imaging to rapidly detect Methicillin-resistant Staphylococcus aureus (MRSA), as well as and using a technique known as surface-enhanced Raman spectroscopy as a diagnostic platform to detect bacteria and antibiotic susceptibility. Ongoing work from the NIAID-supported Antibacterial Resistance Leadership Group (ARLG) includes developing and validating new diagnostics and evaluating the effectiveness of existing platforms. Through the Pneumonia Direct Pilot (PDP) study, ARLG investigators are comparing the results of multiple diagnostics to detect ventilator-acquired pneumonia (VAP) and identify patients who require antibiotic treatment. In addition, in 2018, NIAID awarded several grants under the Partnerships for Development of Clinically Useful Diagnostics for Antimicrobial-Resistant Bacteria program. One research team is working toward developing a device that rapidly and noninvasively diagnoses VAP, identifies its causative microbe, and examines the response of the microbe to empiric antibiotic therapy.

