134 Results
NIH Statement on Preliminary Efficacy Results of Twice-Yearly Lenacapavir for HIV Prevention in Cisgender Women
June 26, 2024
The injectable antiretroviral drug lenacapavir was safe and 100% effective as long-acting HIV pre-exposure prophylaxis (PrEP) among cisgender women in a Phase 3 clinical trial, according to top-line findings released by Gilead Sciences, Inc., the study sponsor. Lenacapavir is administered every six months, making it the most durable HIV prevention method to have shown efficacy in this population.
NIH Releases H5N1 Influenza Research Agenda
June 5, 2024
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, has released its plan for advancing H5N1 influenza basic research and translating those findings into strategies and interventions that can benefit people.
U.S. Clinical Trials Begin for Twice-Yearly HIV Prevention Injection
June 4, 2024
Two clinical trials have launched to examine a novel long-acting form of HIV pre-exposure prophylaxis (PrEP) in cisgender women and people who inject drugs.
Novel Vaccine Concept Generates Immune Responses that Could Produce Multiple Types of HIV Broadly Neutralizing Antibodies
May 30, 2024
Using a combination of cutting-edge immunologic technologies, researchers have successfully stimulated animals’ immune systems to induce rare precursor B cells of a class of HIV broadly neutralizing antibodies (bNAbs). The findings, published today in Nature Immunology, are an encouraging, incremental step in developing a preventive HIV vaccine.
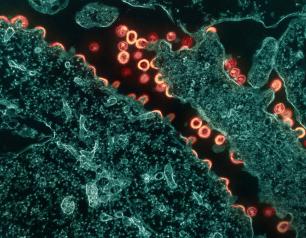
High H5N1 Influenza Levels Found in Mice Given Raw Milk from Infected Dairy Cows
May 24, 2024
Mice administered raw milk samples from dairy cows infected with H5N1 influenza experienced high virus levels in their respiratory organs and lower virus levels in other vital organs, according to findings published in the New England Journal of Medicine. The results suggest that consumption of raw milk by animals poses a risk for H5N1 infection and raises questions about its potential risk in humans.
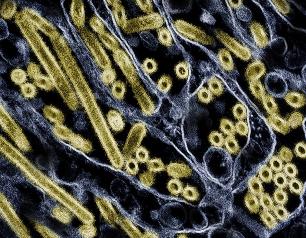
Lower Dose of Mpox Vaccine Is Safe and Generates Six-Week Antibody Response Equivalent to Standard Regimen
April 27, 2024
A dose-sparing intradermal mpox vaccination regimen was safe and generated an antibody response equivalent to that induced by the standard regimen at six weeks (two weeks after the second dose), according to findings presented today at the European Society of Clinical Microbiology and Infectious Diseases Global Congress in Barcelona. The results suggest that antibody responses contributed to the effectiveness of dose-sparing mpox vaccine regimens used during the 2022 U.S. outbreak.
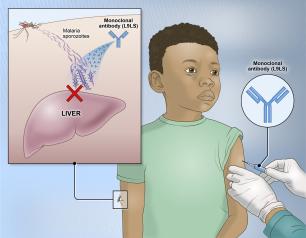
Experimental NIH Malaria Monoclonal Antibody Protective in Malian Children
April 26, 2024
One injected dose of an experimental malaria monoclonal antibody was 77% effective against malaria disease in children in Mali during the country’s six-month malaria season, according to the results of a mid-stage clinical trial. The trial assessed an investigational monoclonal antibody developed by scientists at the National Institutes of Health (NIH), and results appear in The New England Journal of Medicine.
World TB Day 2024 – Yes! We Can End TB!
March 22, 2024
In observance of World Tuberculosis Day (Sunday, March 24), NIAID joins our partners in reaffirming our commitment to ending the tuberculosis (TB) pandemic while honoring the lives lost to TB disease.
NIH Scientists Find Weak Points on Epstein-Barr Virus
March 12, 2024
Studies of interactions between two lab-generated monoclonal antibodies (mAbs) and an essential Epstein-Barr virus (EBV) protein have uncovered targets that could be exploited in designing treatments and vaccines for this extremely common virus. The research was led by Jeffrey I. Cohen, M.D., and colleagues from NIAID.
Children Surpass a Year of HIV Remission after Treatment Pause
March 6, 2024
Four children have remained free of detectable HIV for more than one year after their antiretroviral therapy (ART) was paused to see if they could achieve HIV remission, according to a presentation today at the 2024 Conference on Retroviruses and Opportunistic Infections (CROI) in Denver. The children, who acquired HIV before birth, were enrolled in a clinical trial funded by the National Institutes of Health in which an ART regimen was started within 48 hours of birth and then closely monitored for drug safety and HIV viral suppression.

Long-Acting HIV Treatment Benefits Adults with Barriers to Daily Pill Taking and Adolescents with Suppressed HIV
March 6, 2024
Long-acting, injectable antiretroviral therapy (ART) suppressed HIV replication better than oral ART in people who had previously experienced challenges taking daily oral regimens and was found safe in adolescents with HIV viral suppression, according to two studies presented today at the 2024 Conference on Retroviruses and Opportunistic Infections (CROI) in Denver. Both studies were sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, in collaboration with other NIH institutes.

Semaglutide Reduces Severity of Common Liver Disease in People with HIV
March 5, 2024
A weekly injection of semaglutide was safe and reduced the amount of fat in the liver by 31% in people with HIV and metabolic dysfunction-associated steatotic liver disease (MASLD), according to a presentation today at the 2024 Conference on Retroviruses and Opportunistic Infections (CROI) in Denver. This is the first clinical trial of semaglutide for MASLD in people with HIV.
Vaginal Ring and Oral Pre-Exposure Prophylaxis Found Safe for HIV Prevention Throughout Pregnancy
March 5, 2024
The monthly dapivirine vaginal ring and daily oral pre-exposure prophylaxis (PrEP) with tenofovir disoproxil fumarate and emtricitabine were each found to be safe for HIV prevention among cisgender women who started using one of them in their second trimester of pregnancy, according to findings presented today at the 2024 Conference on Retroviruses and Opportunistic Infections (CROI) in Denver.
Tools Underestimate Cardiovascular Event Risk in People with HIV
March 4, 2024
The elevated cardiovascular disease risk among people with HIV is even greater than predicted by a standard risk calculator in several groups, including Black people and cisgender women, according to analyses from a large international clinical trial primarily funded by the National institutes of Health and presented at the 2024 Conference on Retroviruses and Opportunistic Infections (CROI) in Denver.
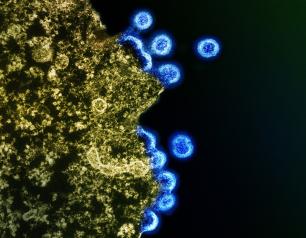
New Antibodies Target “Dark Side” of Influenza Virus Protein
March 1, 2024
Researchers at the National Institutes of Health have identified antibodies targeting a hard-to-spot region of the influenza virus, shedding light on the relatively unexplored “dark side” of the neuraminidase (NA) protein head. The antibodies target a region of the NA protein that is common among many influenza viruses, including H3N2 subtype viruses, and could be a new target for countermeasures. The research, led by scientists at the National Institute of Allergy and Infectious Diseases’ Vaccine Research Center, part of NIH, was published today in Immunity.
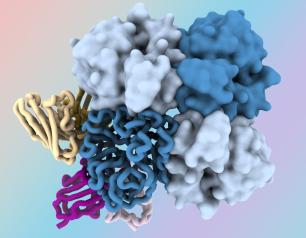
Statement: Long-Acting HIV Treatment Demonstrates Efficacy in People with Challenges Taking Daily Medicine as Prescribed
February 21, 2024
Long-acting antiretroviral therapy (ART) with cabotegravir and rilpivirine was superior in suppressing HIV replication compared to daily oral ART in people who had been unable to maintain viral suppression through an oral daily regimen, according to interim data from a randomized trial. Upon review of these findings, an independent Data and Safety Monitoring Board (DSMB) recommended halting randomization and inviting all eligible study participants to take long-acting ART.
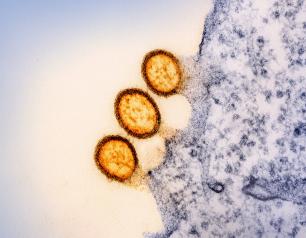
COVID-19 Vaccination and Boosting During Pregnancy Protects Infants for Six Months
February 14, 2024
Women who receive an mRNA-based COVID-19 vaccination or booster during pregnancy can provide their infants with strong protection against symptomatic COVID-19 infection for at least six months after birth. These findings reinforce the importance of receiving both a COVID-19 vaccine and booster during pregnancy to ensure that infants are born with robust protection that lasts until they are old enough to be vaccinated.

NIH-Developed HIV Antibodies Protect Animals in Proof-of-Concept Study
January 17, 2024
Three different HIV antibodies each independently protected monkeys from acquiring simian-HIV (SHIV) in a placebo-controlled proof-of-concept study intended to inform development of a preventive HIV vaccine for people. The antibodies—a human broadly neutralizing antibody and two antibodies isolated from previously vaccinated monkeys—target the fusion peptide, a site on an HIV surface protein that helps the virus fuse with and enter cells.
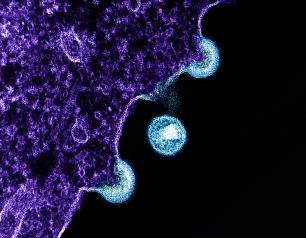
NIH Research Identifies Opportunities to Improve Future HIV Vaccine Candidates
December 14, 2023
An effective HIV vaccine may need to prompt strong responses from immune cells called CD8+ T cells to protect people from acquiring HIV, according to a new study from researchers at the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, and colleagues. The study findings, appearing in Science, draw comparisons between the immune system activity of past HIV vaccine study participants and people with HIV who naturally keep the virus from replicating even in the absence of antiretroviral therapy (ART).

World AIDS Day 2023
December 1, 2023
On this 35th World AIDS Day, the National Institutes of Health (NIH) joins its partners in honoring the lives lost due to the HIV pandemic. For decades, this virus has exacted a tragic toll, affecting people, families, and communities worldwide, threatening social and economic development, and exacerbating stigma, often toward people who already experience discrimination and health disparities.

Clinical Trial to Test Immune Modulation Strategy for Hospitalized COVID-19 Patients Begins
September 22, 2023
A clinical trial has launched to test whether early intensive immune modulation for hospitalized COVID-19 patients with relatively mild illness is beneficial. The placebo-controlled study will enroll approximately 1,500 people at research sites around the world.
Clinical Trial of HIV Vaccine Begins in United States and South Africa
September 20, 2023
A trial of a preventive HIV vaccine candidate has begun enrollment in the United States and South Africa. The Phase 1 trial will evaluate a novel vaccine known as VIR-1388 for its safety and ability to induce an HIV-specific immune response in people. The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, has provided scientific and financial support throughout the lifecycle of this HIV vaccine concept and is contributing funding for this study.
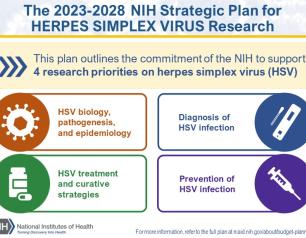
NIH Releases Strategic Plan for Research on Herpes Simplex Virus 1 and 2
September 19, 2023
In response to the persistent health challenges of herpes simplex virus 1 (HSV-1) and HSV-2, an NIH-wide HSV Working Group developed the plan, informed by feedback from more than 100 representatives of the research and advocacy communities and interested public stakeholders. The plan outlines an HSV research framework with four strategic priorities: improving fundamental knowledge of HSV biology, pathogenesis, and epidemiology; accelerating research to improve HSV diagnosis; improving strategies to treat HSV while seeking a curative therapeutic; and, advancing research to prevent HSV infection.
NIH Clinical Trial of Universal Flu Vaccine Candidate Begins
September 15, 2023
Enrollment in a Phase 1 trial of a new investigational universal influenza vaccine candidate has begun at the National Institutes of Health’s Clinical Center in Bethesda, Maryland. The trial is sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the NIH, and will evaluate the investigational vaccine for safety and its ability to elicit an immune response.
NIH Investigates Multidrug-Resistant Bacterium Emerging in Community Settings
September 6, 2023
New “hypervirulent” strains of the bacterium Klebsiella pneumoniae have emerged in healthy people in community settings, prompting a National Institutes of Health research group to investigate how the human immune system defends against infection. After exposing the strains to components of the human immune system in a laboratory “test tube” setting, scientists found that some strains were more likely to survive in blood and serum than others, and that neutrophils (white blood cells) are more likely to ingest and kill some strains than others.