217 Results
An Isolated Viral Load Test May Generate False Positive Results for People Using Long-Acting PrEP
July 23, 2024
A single laboratory-based HIV viral load test used by U.S. clinicians who provide people with long-acting, injectable cabotegravir (CAB-LA) HIV pre-exposure prophylaxis (PrEP) did not reliably detect HIV in a multi-country study. In the study, a single positive viral load test was frequently found to be a false positive result.
Long-Acting Injectable Cabotegravir for HIV Prevention Is Safe in Pregnancy
July 23, 2024
Long-acting injectable cabotegravir (CAB-LA) was safe and well tolerated as HIV pre-exposure prophylaxis (PrEP) before and during pregnancy in the follow-up phase of a global study among cisgender women.
Exploratory Analysis Associates HIV Drug Abacavir with Elevated Cardiovascular Disease Risk in Large Global Trial
July 23, 2024
Current or previous use of the antiretroviral drug (ARV) abacavir was associated with an elevated risk of major adverse cardiovascular events (MACE) in people with HIV, according to an exploratory analysis from a large international clinical trial primarily funded by the National Institutes of Health (NIH).
Features of H5N1 Influenza Viruses in Dairy Cows May Facilitate Infection, Transmission in Mammals
July 8, 2024
A series of experiments with highly pathogenic H5N1 avian influenza (HPAI H5N1) viruses circulating in infected U.S. dairy cattle found that viruses derived from lactating dairy cattle induced severe disease in mice and ferrets when administered via intranasal inoculation.
NIH-Sponsored Trial of Nasal COVID-19 Vaccine Opens
July 1, 2024
A Phase 1 trial testing the safety of an experimental nasal vaccine that may provide enhanced breadth of protection against emerging variants of SARS-CoV-2, the virus that causes COVID-19, is now enrolling healthy adults at three sites in the United States.
NIH-Sponsored Trial of Enterovirus D68 Therapeutic Begins
June 27, 2024
The National Institutes of Health (NIH) is sponsoring a clinical trial to evaluate the safety of an investigational monoclonal antibody to treat enterovirus D68 (EV-D68), which can cause severe respiratory and neurological diseases such as acute flaccid myelitis (AFM) – similar to polio. Scientists are striving to better understand AFM, which has emerged in the United States with spikes in cases every other year, primarily in the late-summer months over the last decade. The U.S. Centers for Disease Control and Prevention (CDC) identified increases in AFM cases in 2014, 2016, and 2018. EV-D68 is a virus of growing public health concern due to its association with the intermittent AFM outbreaks.
NIH Statement on Preliminary Efficacy Results of Twice-Yearly Lenacapavir for HIV Prevention in Cisgender Women
June 26, 2024
The injectable antiretroviral drug lenacapavir was safe and 100% effective as long-acting HIV pre-exposure prophylaxis (PrEP) among cisgender women in a Phase 3 clinical trial, according to top-line findings released by Gilead Sciences, Inc., the study sponsor. Lenacapavir is administered every six months, making it the most durable HIV prevention method to have shown efficacy in this population.
NIAID Discovery Leads to Novel Probiotic for Eczema
June 26, 2024
NIAID research has led to the availability of a new over-the-counter topical eczema probiotic. The probiotic is based on the discovery by scientists at the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, that bacteria present on healthy skin called Roseomonas mucosa can safely relieve eczema symptoms in adults and children.
Infectious H5N1 Influenza Virus in Raw Milk Rapidly Declines with Heat Treatment
June 14, 2024
The amount of infectious H5N1 influenza viruses in raw milk rapidly declined with heat treatment in laboratory research conducted by scientists at the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health. However, small, detectable amounts of infectious virus remained in raw milk samples with high virus levels when treated at 72 degrees Celsius (161.6 degrees Fahrenheit) for 15 seconds—one of the standard pasteurization methods used by the dairy industry.

NIH Releases H5N1 Influenza Research Agenda
June 5, 2024
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, has released its plan for advancing H5N1 influenza basic research and translating those findings into strategies and interventions that can benefit people. The research agenda focuses on four key objectives: increasing understanding of the biology of H5N1 viruses and the factors that influence their ability to transmit and cause disease; developing and evaluating prevention strategies, such as vaccines; advancing existing and novel treatments, including antivirals and monoclonal antibodies; and supporting strategies for detecting H5N1 virus. The NIAID Research Agenda for 2024 H5N1 Influenza – May 2024 aligns with the NIAID role in the federal public health response to the U.S. outbreak of H5N1 influenza in people, dairy cows and other animals.
U.S. Clinical Trials Begin for Twice-Yearly HIV Prevention Injection
June 4, 2024
Two clinical trials have launched to examine a novel long-acting form of HIV pre-exposure prophylaxis (PrEP) in cisgender women and people who inject drugs.
Existing Drug Shows Promise as Treatment for Rare Genetic Disorder
May 30, 2024
A drug approved to treat certain autoimmune diseases and cancers successfully alleviated symptoms of a rare genetic syndrome called autoimmune polyendocrine syndrome type 1 (APS-1).
Novel Vaccine Concept Generates Immune Responses that Could Produce Multiple Types of HIV Broadly Neutralizing Antibodies
May 30, 2024
Using a combination of cutting-edge immunologic technologies, researchers have successfully stimulated animals’ immune systems to induce rare precursor B cells of a class of HIV broadly neutralizing antibodies (bNAbs). The findings, published today in Nature Immunology, are an encouraging, incremental step in developing a preventive HIV vaccine.
Introducing Peanut in Infancy Prevents Peanut Allergy into Adolescence
May 28, 2024
Feeding children peanut products regularly from infancy to age 5 years reduced the rate of peanut allergy in adolescence by 71%, even when the children ate or avoided peanut products as desired for many years. These new findings, from a study sponsored and co-funded by the National Institutes of Health’s National Institute of Allergy and Infectious Diseases (NIAID), provide conclusive evidence that achieving long-term prevention of peanut allergy is possible through early allergen consumption. The results were published today in the journal NEJM Evidence.

High H5N1 Influenza Levels Found in Mice Given Raw Milk from Infected Dairy Cows
May 24, 2024
Mice administered raw milk samples from dairy cows infected with H5N1 influenza experienced high virus levels in their respiratory organs and lower virus levels in other vital organs, according to findings published in the New England Journal of Medicine. The results suggest that consumption of raw milk by animals poses a risk for H5N1 infection and raises questions about its potential risk in humans.
NIH Study Shows Chronic Wasting Disease Unlikely to Move from Animals to People
May 17, 2024
A new study of prion diseases, using a human cerebral organoid model, suggests there is a substantial species barrier preventing transmission of chronic wasting disease (CWD) from cervids—deer, elk and moose—to people. The findings, from National Institutes of Health scientists and published in Emerging Infectious Diseases, are consistent with decades of similar research in animal models at the NIH’s National Institute of Allergy and Infectious Diseases (NIAID).
Lower Dose of Mpox Vaccine Is Safe and Generates Six-Week Antibody Response Equivalent to Standard Regimen
April 27, 2024
A dose-sparing intradermal mpox vaccination regimen was safe and generated an antibody response equivalent to that induced by the standard regimen at six weeks (two weeks after the second dose), according to findings presented today at the European Society of Clinical Microbiology and Infectious Diseases Global Congress in Barcelona. The results suggest that antibody responses contributed to the effectiveness of dose-sparing mpox vaccine regimens used during the 2022 U.S. outbreak.
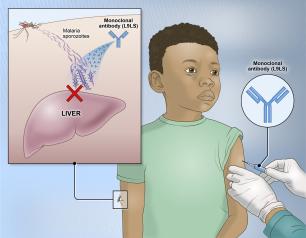
Experimental NIH Malaria Monoclonal Antibody Protective in Malian Children
April 26, 2024
One injected dose of an experimental malaria monoclonal antibody was 77% effective against malaria disease in children in Mali during the country’s six-month malaria season, according to the results of a mid-stage clinical trial. The trial assessed an investigational monoclonal antibody developed by scientists at the National Institutes of Health (NIH), and results appear in The New England Journal of Medicine.
World TB Day 2024 – Yes! We Can End TB!
March 22, 2024
In observance of World Tuberculosis Day (Sunday, March 24), NIAID joins our partners in reaffirming our commitment to ending the tuberculosis (TB) pandemic while honoring the lives lost to TB disease.
NIH Scientists Find Weak Points on Epstein-Barr Virus
March 12, 2024
Studies of interactions between two lab-generated monoclonal antibodies (mAbs) and an essential Epstein-Barr virus (EBV) protein have uncovered targets that could be exploited in designing treatments and vaccines for this extremely common virus. The research was led by Jeffrey I. Cohen, M.D., and colleagues from NIAID.
Children Surpass a Year of HIV Remission after Treatment Pause
March 6, 2024
Four children have remained free of detectable HIV for more than one year after their antiretroviral therapy (ART) was paused to see if they could achieve HIV remission, according to a presentation today at the 2024 Conference on Retroviruses and Opportunistic Infections (CROI) in Denver.

Long-Acting HIV Treatment Benefits Adults with Barriers to Daily Pill Taking and Adolescents with Suppressed HIV
March 6, 2024
Long-acting, injectable antiretroviral therapy (ART) suppressed HIV replication better than oral ART in people who had previously experienced challenges taking daily oral regimens and was found safe in adolescents with HIV viral suppression, according to two studies presented today at the 2024 Conference on Retroviruses and Opportunistic Infections (CROI) in Denver. Both studies were sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, in collaboration with other NIH institutes.

Semaglutide Reduces Severity of Common Liver Disease in People with HIV
March 5, 2024
A weekly injection of semaglutide was safe and reduced the amount of fat in the liver by 31% in people with HIV and metabolic dysfunction-associated steatotic liver disease (MASLD), according to a presentation today at the 2024 Conference on Retroviruses and Opportunistic Infections (CROI) in Denver. This is the first clinical trial of semaglutide for MASLD in people with HIV.
Vaginal Ring and Oral Pre-Exposure Prophylaxis Found Safe for HIV Prevention Throughout Pregnancy
March 5, 2024
The monthly dapivirine vaginal ring and daily oral pre-exposure prophylaxis (PrEP) with tenofovir disoproxil fumarate and emtricitabine were each found to be safe for HIV prevention among cisgender women who started using one of them in their second trimester of pregnancy, according to findings presented today at the 2024 Conference on Retroviruses and Opportunistic Infections (CROI) in Denver.
Tools Underestimate Cardiovascular Event Risk in People with HIV
March 4, 2024
The elevated cardiovascular disease risk among people with HIV is even greater than predicted by a standard risk calculator in several groups, including Black people and cisgender women, according to analyses from a large international clinical trial primarily funded by the National institutes of Health and presented at the 2024 Conference on Retroviruses and Opportunistic Infections (CROI) in Denver.