217 Results
Statement—U.S. Clinical Trial Results Show Novavax Vaccine is Safe and Prevents COVID-19
June 14, 2021
Results from the PREVENT-19 clinical trial show that the investigational NVX-CoV2373 vaccine demonstrated 90.4% efficacy in preventing symptomatic COVID-19 disease.
Clinical Trial Evaluating Mixed COVID-19 Vaccine Schedules Begins
June 1, 2021
The National Institutes of Health has started a Phase 1/2 clinical trial in which adult volunteers who have been fully vaccinated against COVID-19 will receive booster doses of different COVID-19 vaccines to determine the safety and immunogenicity of mixed boosted regimens. The National Institute of Allergy and Infectious Diseases (NIAID), part of NIH, is leading and funding the study through the Infectious Diseases Clinical Research Consortium, a clinical trials network that encompasses the Institute’s long-standing Vaccine and Treatment Evaluation Units (VTEUs).
NIH Launches Clinical Trial of Universal Influenza Vaccine Candidate
June 1, 2021
A first-in-human, Phase 1 trial assessing the safety and immunogenicity of an investigational nanoparticle influenza vaccine designed to provide long-lasting protection against multiple flu virus strains has begun at the National Institutes of Health Clinical Center in Bethesda, Maryland. Healthy participants 18 to 50 years old will receive either a licensed seasonal influenza vaccine or the experimental vaccine, FluMos-v1.

NIH Scientists Find that Salmonella Use Intestinal Epithelial Cells to Colonize the Gut
May 26, 2021
The immune system’s attempt to eliminate Salmonella bacteria from the gastrointestinal (GI) tract instead facilitates colonization of the intestinal tract and fecal shedding, according to National Institutes of Health scientists. The study, published in Cell Host & Microbe, was conducted by National Institute of Allergy and Infectious Diseases (NIAID) scientists at Rocky Mountain Laboratories in Hamilton, Montana.
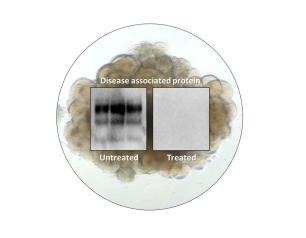
Gene Therapy Restores Immune Function in Children with Rare Immunodeficiency
May 11, 2021
An investigational gene therapy can safely restore the immune systems of infants and children who have a rare, life-threatening inherited immunodeficiency disorder, according to research supported in part by the National Institutes of Health.

NIH Statement on World Asthma Day 2021
May 5, 2021
On World Asthma Day, the National Institutes of Health reaffirms its commitment to research to improve the lives of people with asthma. More than 25 million people in the United States have asthma, including 5.1 million children, according to the Centers for Disease Control and Prevention. This chronic lung disease can reduce quality of life, contributes to considerable emotional and financial stress, and is a major contributing factor to missed time from school and work.
Trial of Existing Antibiotic for Treating Staphylococcus aureus Bacteremia Begins
April 27, 2021
A clinical trial to test the antibiotic dalbavancin for safety and efficacy in treating complicated Staphylococcus aureus (S. aureus) bacteremia has begun. The trial will enroll 200 adults hospitalized with complicated S. aureus infection at approximately 20 trial sites around the United States. The trial is being sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health.
COVID-19 Vaccine Responses to be Studied in People with Immune Deficits
April 23, 2021
A study assessing how people with immune system deficiencies or dysregulations respond to COVID-19 vaccination has begun enrolling participants at the National Institutes of Health Clinical Center in Bethesda, Maryland. The single-site study is led by researchers from the National Institute of Allergy and Infectious Diseases (NIAID) and aims to enroll 500 people, 400 with primary or secondary immune system disorders and 100 without such conditions.
NIH Establishes New Childhood Asthma Clinical Research Network
April 23, 2021
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, has awarded $10 million in first-year funding to establish a clinical research network called Childhood Asthma in Urban Settings (CAUSE). This nationwide network will conduct observational studies and clinical trials to improve understanding of asthma and develop treatment and prevention approaches tailored to children of low-income families living in urban communities. NIAID intends to provide approximately $70 million over seven years to support the CAUSE network.
Clinical Trial of Therapeutics for Severely Ill Hospitalized COVID-19 Patients Begins
April 22, 2021
A new Phase 3 trial to test the safety and efficacy of therapeutics for COVID-19 has begun enrolling patients hospitalized with life-threatening cases of COVID-19, including those with acute respiratory failure.
Statement—Large NIH Clinical Trial Will Test Polyclonal Antibody Therapeutic for COVID-19
April 21, 2021
A Phase 2/3 trial to evaluate a new fully-human polyclonal antibody therapeutic targeted to SARS-CoV-2, called SAB-185, has begun enrolling non-hospitalized people with mild or moderate cases of COVID-19. The trial, ACTIV-2, is sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health. The therapeutic was developed by SAB Biotherapeutics, Inc. (Sioux Falls, South Dakota).
Experimental Antiviral for COVID-19 Effective in Hamster Study
April 16, 2021
The experimental antiviral drug MK-4482 significantly decreased levels of virus and disease damage in the lungs of hamsters treated for SARS-CoV-2 infection, according to a new study from National Institutes of Health scientists. SARS-CoV-2 is the virus that causes COVID-19. MK-4482, delivered orally, is now in human clinical trials. Remdesivir, an antiviral drug already approved by the U.S. Food and Drug Administration for use against COVID-19, must be provided intravenously, making its use primarily limited to clinical settings.
NIAID Funds New Influenza Research Network
April 14, 2021
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, has established a network of research sites to study the natural history, transmission and pathogenesis of influenza and provide an international research infrastructure to address influenza outbreaks.
Statement—NIH Closes Enrollment in Trial Comparing COVID-19 Treatment Regimens
April 14, 2021
The National Institute of Allergy and Infectious Diseases (NIAID), one of the National Institutes of Health, today announced that the fourth iteration of the Adaptive COVID-19 Treatment Trial (ACTT-4) has closed to enrollment because the study met pre-defined futility criteria indicating that neither treatment regimen studied is likely significantly better than the other.
NIH Experts Discuss Post-Acute COVID-19
April 13, 2021
Many people who have COVID-19 make a full recovery and return to their baseline state of health; however, some people have symptoms or other sequelae weeks or months after initial SARS-CoV-2 infection. These heterogeneous symptoms were the subject of the virtual “Workshop on Post-acute Sequelae of COVID-19” hosted on Dec. 2 and 4, 2020, by the National Institute of Allergy and Infectious Diseases (NIAID), in collaboration with other institutes and centers of the National Institutes of Health. A paper published recently in Annals of Internal Medicine describes the workshop.

NIH Trial of Anti-CD14 Antibody to Treat COVID-19 Respiratory Disease Begins
April 13, 2021
A clinical trial testing the safety and efficacy of an investigational monoclonal antibody for treating people who are hospitalized with respiratory disease and low blood oxygen due to infection with SARS-CoV-2, the virus that causes COVID-19, has begun. The Phase 2 trial, called the COVID-19 anti-CD14 Treatment Trial (CaTT), is sponsored and funded by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health.
NIH Experts Call for Accelerated Research to Address Concurrent HIV and COVID-19 Pandemics
April 8, 2021
The COVID-19 pandemic is affecting people with or at risk for HIV both indirectly, by interfering with HIV treatment and prevention services, and directly, by threatening individual health. An effective response to these dual pandemics requires unprecedented collaboration to accelerate basic and clinical research, as well as implementation science to expeditiously introduce evidence-based strategies into real-world settings. This message comes from a review article co-authored by Anthony S.
NIH Begins Study of Allergic Reactions to Moderna, Pfizer-BioNTech COVID-19 Vaccines
April 7, 2021
A clinical trial is underway to determine whether people who are highly allergic or have a mast cell disorder are at increased risk for an immediate, systemic allergic reaction to the Moderna or Pfizer-BioNTech COVID-19 vaccines. A systemic allergic reaction to a vaccine occurs in one or more parts of the body beyond the injection site. If such an allergic reaction occurs in study participants, investigators will assess whether the reactions are more frequent in participants who are highly allergic or have a mast cell disorder than in participants with no allergic history.
NIH Clinical Trial Evaluating Moderna COVID-19 Variant Vaccine Begins
March 31, 2021
An investigational vaccine designed to protect against the B.1.351 SARS-CoV-2 variant has been administered as part of a new Phase 1 clinical trial evaluating the vaccine candidate’s safety and immunogenicity in adult volunteers. The vaccine, known as mRNA-1273.351, was developed by the biotechnology company ModernaTX, Inc., based in Cambridge, Massachusetts. The trial is led and funded by NIAID. The trial will enroll approximately 210 healthy adult volunteers at four clinical research sites in the United States that are part of the NIAID-funded Infectious Diseases Clinical Research Consortium (IDCRC).
T Cells Recognize Recent SARS-CoV-2 Variants
March 30, 2021
When variants of SARS-CoV-2 (the virus that causes COVID-19) emerged in late 2020, concern arose that they might elude protective immune responses generated by prior infection or vaccination, potentially making re-infection more likely or vaccination less effective. To investigate this possibility, researchers from the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, and colleagues analyzed blood cell samples from 30 people who had contracted and recovered from COVID-19 prior to the emergence of virus variants.
NIAID Statement on AstraZeneca Vaccine
March 23, 2021
NIAID urges AstraZeneca to work with the Data and Safety Monitoring Board (DSMB) to review the efficacy data from its COVID-19 vaccine clinical trial and ensure the most accurate, up-to-date efficacy data be made public as quickly as possible.
Statement—Investigational AstraZeneca Vaccine Prevents COVID-19
March 22, 2021
Results from a large clinical trial in the United States and South America indicate that AstraZeneca’s COVID-19 vaccine, AZD1222, is well-tolerated and protects against symptomatic COVID-19 disease, including severe disease or hospitalization.
NIH Study of Early Predictors, Mechanisms of Food Allergy and Eczema has Begun
March 19, 2021
A study to identify prenatal and early childhood markers of high risk for food allergy and atopic dermatitis, or eczema, as well as biological pathways that lead to these conditions, has begun. The observational study of children from birth to age 3 years will examine the origins of allergic disease by integrating interdisciplinary analyses of data from more than 260 biological and environmental samples and survey responses from each of 2,500 families.

NIH Awards Grants to Support Bacteriophage Therapy Research
March 11, 2021
NIAID has awarded $2.5 million in grants to 12 institutes around the world to support research on bacteriophage therapy.
NIH Scientists Use Human Cerebral Organoid to Test Drug for Deadly Brain Disease
March 9, 2021
After establishing a human cerebral organoid system to study CJD, NIAID researchers have developed the model to screen drugs for potential CJD treatment.