Malaria Immunology Section
Carole Long, Ph.D.
Chief, Malaria Immunology Section
Director, PATH Malaria Vaccine Initiative Standard Membrane Feeding Assay-Reference Center
Director, USAID Growth Inhibition Assay–Reference Center

Major Areas of Research
- Studying the acquisition of immunity to malaria in those living in malaria-endemic areas
- Investigating the process of transmission of malaria in the field
- Identifying and evaluating possible new malaria vaccine candidates, focusing on the erythrocytic and sexual stages of malaria infection
- Standardization and application of an in vitro parasite growth inhibition assay (GIA) and a standard membrane feeding assay (SMFA) for testing antibodies to Plasmodium falciparum erythrocytic and sexual stages, respectively
Program Description
Research in the Malaria Immunology Section focuses on analysis of the interface between the malaria parasite and the immune system of the vertebrate host. It is known that those living in malaria-endemic areas progressively acquire resistance to this infection, although this takes many years to attain. We have also known that pioneering studies going back to the early 1960s have established that antibodies from adults in endemic areas, when passively transferred to children with malaria, can drive down parasitemias. However, we still do not know the antigenic targets of those antibodies, nor do we know the effector mechanisms involved in the reduction of parasite burdens. In addition, we have only limited knowledge of the parasite sexual stages and their transmission through mosquitoes in the field. Our studies are directed toward a better understanding of these complex and important parasites, as well as identification and evaluation of possible candidate antigens for a malaria vaccine.
We are studying the acquisition of clinical immunity in children living in malaria-endemic areas and investigating the impact of hemoglobin polymorphisms such as sickle cell trait on malaria incidence and disease. We have a long-standing collaboration with investigators in Mali, and in 2008, we established a new field site in Kenieroba, Mali. We are characterizing malaria-specific antibody responses in children and adults to both erythrocytic and sexual stages of parasite development. Antibodies to these stages are being characterized for their functional activity against parasites using a number of different assays. Moreover, these assays are being used to evaluate various parasite-encoded proteins as potential malaria vaccine candidates.
Because the process of transmission in the field is not well understood, we initiated a new project in 2013 for a comprehensive assessment of dynamics of erythrocyte and sexual stage parasites and immunity and sexual stage parasites in our field site in Mali, and we are currently determining possible targets of antibodies that might block transmission to mosquitoes. Understanding the assays that are utilized to measure transmission blocking, the parasite antigens that might be effective vaccine candidates against sexual stage parasites, and the process of malaria transmission in the field will aid in efforts to develop a vaccine to block transmission of infection as a novel strategy for control of this disease.
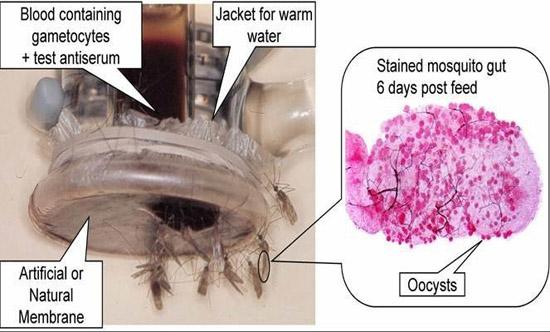
Mosquito membrane feeding assay. Mosquitoes are fed on blood containing the cultured sexual stages of malaria parasites. One week later, the mosquito mid-guts are dissected and stained. The red circular oocysts on the right contain infectious stages of the parasites called sporozoites, which migrate to the mosquito salivary glands.
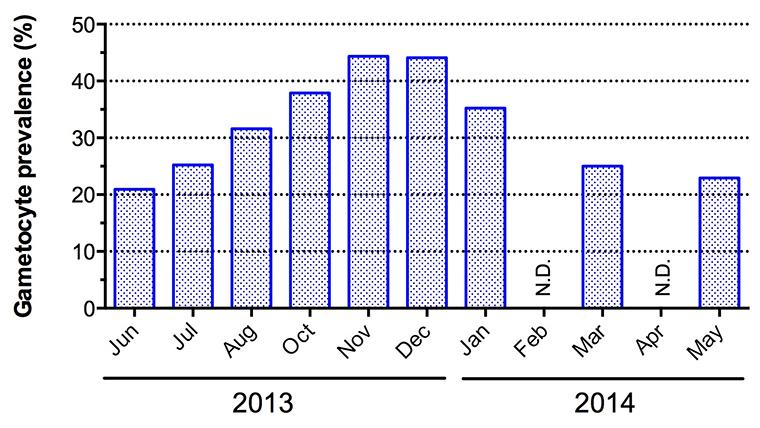
P. falciparum gametocyte prevalence detected by Pfs25-based RT-PCR in Kenieroba, Mali, from June 2013 to May 2014. Five hundred people in all age ranges were followed for their total parasite and gametocyte positivity for one year. N.D.; not determined yet
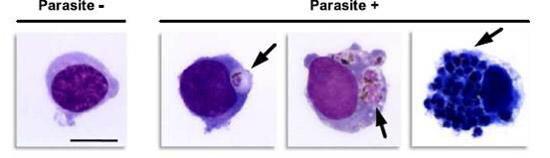
Human monocytes phagocytosing red cells infected with malaria parasites. Cell on the left has no parasite, while the three cells on the right have engulfed different numbers of parasites. (Lambert L et al., manuscript submitted).
Biography
Education
Ph.D., University of Pennsylvania
Dr. Long received her Ph.D. in microbiology and immunology from the University of Pennsylvania and also did postdoctoral training there. Before joining NIAID in 1999, Dr. Long was a professor of microbiology and immunology at Hahnemann University School of Medicine (now Drexel University) in Philadelphia. She has served as president of the American Society for Tropical Medicine and Hygiene and chair of the Tropical Medicine and Parasitology Study Section. Her lab’s work focuses on immune responses to malaria parasites, particularly in those living in malaria-endemic areas, and also on identification and evaluation of possible candidate antigens for malaria vaccines.
Selected Publications
Lopera-Mesa TM, Doumbia S, Konaté D, Anderson JM, Doumbouya M, Keita AS, Diakité SA, Traoré K, Krause MA, Diouf A, Moretz SE, STullo G, Miura K, Gu W, Fay MP, Taylor SM, Long CA, Diakité M, Fairhurst RM. Impact of red blood cell variants on childhood malaria in Mali: a prospective cohort study. Lancet Haematol. 2015 Apr 1;2(4):e140-e149.
Douglas AD, Baldeviano GC, Lucas CM, Lugo-Roman LA, Crosnier C, Bartholdson SJ, Diouf A, Miura K, Lambert LE, Ventocilla JA, Leiva KP, Milne KH, Illingworth JJ, Spencer AJ, Hjerrild KA, Alanine DG, Turner AV, Moorhead JT, Edgel KA, Wu Y, Long CA, Wright GJ, Lescano AG, Draper SJ. A PfRH5-based vaccine is efficacious against heterologous strain blood-stage Plasmodium falciparum infection in aotus monkeys. Cell Host Microbe. 2015 Jan 14;17(1):130-9.
Lambert LH, Bullock JL, Cook ST, Miura K, Garboczi DN, Diakite M, Fairhurst RM, Singh K, Long CA. Antigen reversal identifies targets of opsonizing IgGs against pregnancy-associated malaria. Infect Immun. 2014 Nov;82(11):4842-53.
Miura K, Takashima E, Deng B, Tullo G, Diouf A, Moretz SE, Nikolaeva D, Diakite M, Fairhurst RM, Fay MP, Long CA, Tsuboi T.Functional comparison of Plasmodium falciparum transmission-blocking vaccine candidates by the standard membrane-feeding assay. Infect Immun. 2013 Dec;81(12):4377-82.
Zeituni AE, Miura K, Diakite M, Doumbia S, Moretz SE, Diouf A, Tullo G, Lopera-Mesa TM, Bess CD, Mita-Mendoza NK, Anderson JM, Fairhurst RM, Long CA. Effects of age, hemoglobin type and parasite strain on IgG recognition of Plasmodium falciparum-infected erythrocytes in Malian children. PLoS One. 2013 Oct 4;8(10):e76734.
Miura K, Herrera R, Diouf A, Zhou H, Mu J, Hu Z, MacDonald NJ, Reiter K, Nguyen V, Shimp RL Jr, Singh K, Narum DL, Long CA, Miller LH. Overcoming allelic specificity by immunization with five allelic forms of Plasmodium falciparum apical membrane antigen 1. Infect Immun. 2013 May;81(5):1491-501.
Research Group

Back Row: Yonas Gebremicale, Luwen Zhou, Phuong Thao Pham, Ragavan Varadharajan Suresh, Margaret Smith, Alex Huber, Bingbing Deng, Ababacar Diouf
Front Row: Carole Long, Kazutoyo Miura

