Viral Pathogenesis and Vaccine Section
Established in 1981
Malcolm A. Martin, M.D.
Chief, Laboratory of Molecular Microbiology
Chief, Viral Pathogenesis and Vaccine Section

Major Areas of Research
- Investigates the contribution of individual HIV-1 and SIV genes inducing immunodeficiency in SHIV infected rhesus macaques
- Studies the use of broadly-acting anti-HIV-1 neutralizing antibodies (bNAbs) to treat SHIV infected rhesus macaques
- Assesses the role of bNAbs in preventing SHIV acquisition of NHPs by pre-exposure immunoprophylaxis
- Develops vaccine regimens targeting inferred germline B cell precursors of anti-HIV bNAbs
Program Description
The Viral Pathogenesis and Vaccine Section (VPVS) develops and uses SIV and SIV/HIV chimeric viruses (SHIVs) as surrogates of HIV-1 to investigate virus-induced immunopathogenesis and to develop effective prophylactic vaccines in nonhuman primate models. Toward this end, we have constructed X4- and R5-tropic SHIVs that durably infect macaques and cause systemic depletion of CD4+ T cells in rhesus monkeys, resulting in clinical disease. Recent work has focused on 1) the use of anti-HIV-1 bNAbs to prevent and treat SHIV infections of rhesus macaques and 2) the development of vaccine regimens targeting inferred germline B cell precursors of anti-HIV bNAbs.
Biography
Education
M.D., 1962, Yale University School of Medicine, New Haven, CTDr. Martin received an M.D. from Yale University School of Medicine in 1962 and, following two years of clinical training in internal medicine at the University of Rochester, joined NIH as a research associate. He initially investigated the replication and gene regulation of SV40 and polyomaviruses and subsequently studied endogenous murine and human retroviral sequences. Since 1984, his research program has focused on HIV. Dr. Martin was appointed chief of the Laboratory of Molecular Microbiology when it was established in 1981. He is a member of the National Academy of Sciences and the recipient of numerous scientific awards.
Selected Publications
Escolano A, Gristick HB, Gautam R, DeLaitsch AT, Abernathy ME, Yang Z, Wang H, Hoffmann MAG, Nishimura Y, Wang Z, Koranda N, Kakutani LM, Gao H, Gnanapragasam PNP, Raina H, Gazumyan A, Cipolla M, Oliveira TY, Ramos V, Irvine DJ, Silva M, West AP Jr, Keeffe JR, Barnes CO, Seaman MS, Nussenzweig MC, Martin MA, Bjorkman PJ. Sequential immunization of macaques elicits heterologous neutralizing antibodies targeting the V3-glycan patch of HIV-1 Env. Sci Transl Med. 2021 Nov 24;13(621):eabk1533.
Nishimura Y, Donau OK, Dias J, Ferrando-Martinez S, Jesteadt E, Sadjadpour R, Gautam R, Buckler-White A, Geleziunas R, Koup RA, Nussenzweig MC, Martin MA. Immunotherapy during the acute SHIV infection of macaques confers long-term suppression of viremiav. J Exp Med. 2021 Jan 4;218(1):e20201214.
Nishimura Y, Francis JN, Donau OK, Jesteadt E, Sadjadpour R, Smith AR, Seaman MS, Welch BD, Martin MA, Kay MS. Prevention and treatment of SHIVAD8 infection in rhesus macaques by a potent d-peptide HIV entry inhibitor. Proc Natl Acad Sci U S A. 2020 Sep 8;117(36):22436-22442.
Escolano A, Gristick HB, Abernathy ME, Merkenschlager J, Gautam R, Oliveira TY, Pai J, West AP Jr, Barnes CO, Cohen AA, Wang H, Golijanin J, Yost D, Keeffe JR, Wang Z, Zhao P, Yao KH, Bauer J, Nogueira L, Gao H, Voll AV, Montefiori DC, Seaman MS, Gazumyan A, Silva M, McGuire AT, Stamatatos L, Irvine DJ, Wells L, Martin MA, Bjorkman PJ, Nussenzweig MC. Immunization expands B cells specific to HIV-1 V3 glycan in mice and macaques. Nature. 2019 Jun;570(7762):468-473.
Gautam R, Nishimura Y, Gaughan N, Gazumyan A, Schoofs T, Buckler-White A, Seaman MS, Swihart BJ, Follmann DA, Nussenzweig MC, Martin MA. A single injection of crystallizable fragment domain-modified antibodies elicits durable protection from SHIV infection. Nat Med. 2018 May;24(5):610-616.
Nishimura Y, Gautam R, Chun TW, Sadjadpour R, Foulds KE, Shingai M, Klein F, Gazumyan A, Golijanin J, Donaldson M, Donau OK, Plishka RJ, Buckler-White A, Seaman MS, Lifson JD, Koup RA, Fauci AS, Nussenzweig MC, Martin MA. Early antibody therapy can induce long-lasting immunity to SHIV. Nature. 2017 Mar 23;543(7646):559-563.
Research Group
Yoshiaki Nishimura, Ph.D., Staff Scientist
Bernard Lafont, Ph.D., Staff Scientist
Rajeev Gautam, Ph.D., Staff Scientist
Masashi Shingai, Ph.D., Research Fellow
Wendy Lee, B.S., Biologist
Olivia Donau, B.A., Biologist
Reza Sadjadpour, M.S., Biologist
Images
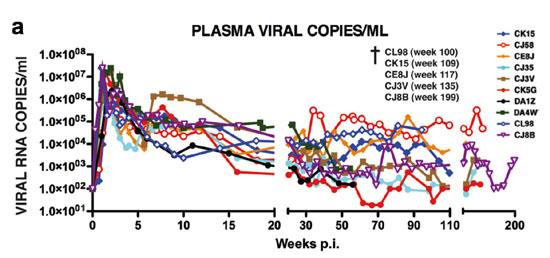
Figure 2A. Plasma Viral Copies / ML
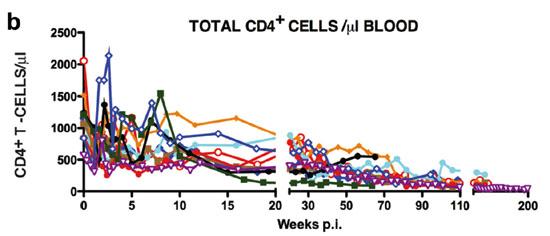
Figure 2: The levels of plasma viremia (a) and absolute numbers of peripheral CD4+ T lymphocytes (b) in rhesus macaques inoculated intravenously with the R5-tropic SHIVAD8.
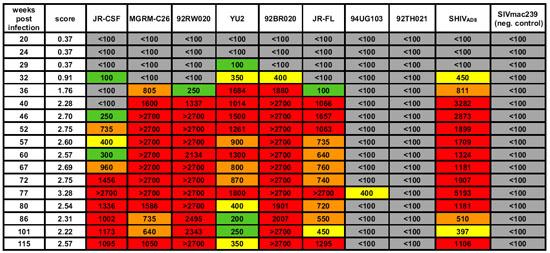
Figure 3: Development of potent broadly cross-clade neutralizing antibodies directed against heterologous HIV-1 strains by a macaque (Rh CE8J) inoculated with the R5-tropic SHIVAD8. Titers in each cell are color coded as follows: gray, IC50 <1:100; green, 1:100< IC50 <1:300; yellow, 1:300< IC50 <1:500; orange, 1:500< IC50 <1:1000; red, IC50 >1:1000.
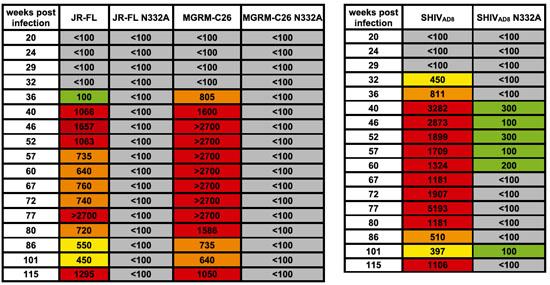
Figure 4: The cross-reacting anti-HIV-1 neutralizing activity generated in macaque CE8J is directed against the gp120 N332 glycan epitope. Plasma samples collected at serial time points were tested for neutralizing activity against JR-CSF, MGRM-C26, and SHIVAD8 pseudovirus variants containing an N332A mutation. Cells are color coded as follows: gray, IC50 <1:100; green, 1:100< IC50 <1:300; yellow, 1:300< IC50 <1:600; orange, 1:600< IC50 <1:1000; red, IC50 >1:1000.

