Immunobiology Section
Alan Sher, Ph.D.
NIH Distinguished Investigator, Laboratory of Parasitic Diseases

Major Areas of Research
- Mechanisms of host resistance and immune regulation in parasitic and mycobacterial infection
- Role of innate pathogen recognition in the initiation of adaptive immunity and in CD4+ T-cell subset effector choice
- Regulatory pathways limiting pathogen-induced Th1 immunopathology
- Immunotherapeutic approaches to the treatment of infectious disease
Program Description
The Immunobiology Section studies host resistance and immune regulation in parasitic and other infections of global importance. The ultimate goal of this work is immunologic disease intervention in the form of immunotherapy or vaccination. At the same time, our research on the host response to infection has provided basic insights into the effector functions and regulatory mechanisms used by the vertebrate immune system and in the role of innate pathogen recognition in these processes. Much of the work of the section is focused on the immunologic analysis in murine models of diseases induced by parasitic and bacterial agents (e.g., Toxoplasma gondii, Mycobacterium spp.), although the group is also engaged in several major clinical collaborations. Recent projects have focused on necrotic cell death pathways as targets for host directed therapy of M. tuberculosis (Mtb) and interactions of the microbiome with Mtb infection and treatment.
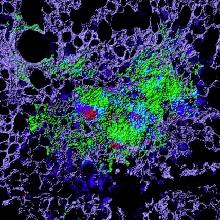
Cellular necrosis induced by Mycobacterium tuberculosis (Mtb) infection. This process causes both tissue damage and dissemination of the pathogen and is thus an important target for therapeutic intervention. The image shown is an Mtb granuloma within the lung of an infected mouse. The green staining identifies the necrotic tissue core within the pulmonary matrix (violet). The red and blue staining cells are the bacteria and host macrophages, respectively. Using an inhibitor of a cell death mechanism known as ferroptosis we are able to markedly reduce the tissue necrosis observed.
Biography
Education
Ph.D., University of California, San Diego
Dr. Sher received his Ph.D. from the University of California, San Diego, and did his postdoctoral training in the Division of Parasitology at the National Institute for Medical Research in Mill Hill, London. In 1980, after several years as a research associate and then assistant professor in the department of pathology at Harvard Medical School, he joined NIAID as a section chief in the Laboratory of Parasitic Diseases (LPD). Sher became chief of LPD in 2003 and was promoted to NIH Distinguished Investigator in 2011. In June 2022, he closed his laboratory research program to focus primarily on mentoring activities.
Awards
- Bonazinga Award (Society for Leukocyte Biology)
- Bailey K. Ashford Medal (The American Society of Tropical Medicine and Hygiene)
- U.S. PHS Superior Service Award
- NIH Director’s Mentoring Award
- International Interferon and Cytokine Society William E. Paul Award
Memberships
- Fellow, American Academy of Microbiology
- Fellow, American Association for the Advancement of Science
- Member, Brazilian Academy of Sciences
Editorial Boards
- Faculty of 1000 (Section Head, Immunity to Infections)
- mBio (American Society of Microbiology Journal)
- Immunology and Cell Biology
- The Journal of Experimental Medicine (Emeritus Editor)
Selected Publications
Hilligan KL, Namasivayam S, Clancy CS, O'Mard D, Oland SD, Robertson SJ, Baker PJ, Castro E, Garza NL, Lafont BAP, Johnson R, Ronchese F, Mayer-Barber KD, Best SM, Sher A. Intravenous administration of BCG protects mice against lethal SARS-CoV-2 challenge. J. Exp. Med. 2022 Feb 7;219(2).
Amaral EP, Costa DL, Namasivayam S, Riteau N, Kamenyeva O, Mittereder L, Mayer-Barber KD, Andrade BB, Sher A. A major role for ferroptosis in Mycobacterium tuberculosis induced cell death and tissue necrosis. J Exp Med. 2019 Mar 4;216(3):556-570.
Namasivayam S, Maiga M, Yuan W, Thovarai V, Costa DL, Mittereder LR, Wipperman MF, Glickman MS, Dzutsev A, Trinchieri G, Sher A. Longitudinal profiling reveals a persistent intestinal dysbiosis triggered by conventional anti-tuberculosis therapy. Microbiome. 2017 Jul 7;5(1):71.
Iwamura C, Bouladoux N, Belkaid Y, Sher A, Jankovic D. Sensing of the microbiota by NOD1 in mesenchymal stromal cells regulates murine hematopoiesis. Blood. 2017 Jan 12;129(2):171-176.
Kugler DG, Flomerfelt FA, Costa DL, Laky K, Kamenyeva O, Mittelstadt PR, Gress RE, Rosshart SP, Rehermann B, Ashwell JD, Sher A, Jankovic D. Systemic toxoplasma infection triggers a long-term defect in the generation and function of naive T lymphocytes. J Exp Med. 2016 Dec 12;213(13):3041-3056.
Mayer-Barber KD, Andrade BB, Oland SD, Amaral EP, Barber DL, Gonzales J, Derrick SC, Shi R, Kumar NP, Wei W, Yuan X, Zhang G, Cai Y, Babu S, Catalfamo M, Salazar AM, Via LE, Barry CE 3rd, Sher A. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature. 2014 Jul 3;511(7507):99-103.
Research Group

Top Row: Edu Amaral, Diego Costa, Ranjani Namasivayam, Sanjay Gautam, Logan Fisher Middle Row: Gil Bundoc, Roberta Carter, Alan Sher, Kate Aberman, Kerry Hilligan, Oluwatoyin Adeleke
Bottom Row: Effie Reyes, Dragana Jankovic, Claire Conarroe
Accomplishments
- Demonstration of the role of TLR 11-recognition of parasite profilin in the induction of IL-12 during T. gondii infection
- Identification of the T2 ribonuclease Omega-1 as the schistosome egg component responsible for conditioning dendritic cells (DC) to trigger Th2 responses
- Elucidation of endoplasmic reticulum fusion with parasitophorous vacuoles as a mechanism of cross-presentation in T. gondii-infected DC
- Identification of IL-10-producing Th1 cells as major regulators of immunopathology in T. gondii infection
- Discovery of major roles for the GTPase Irgm1(LRG47) in the regulation of hematopoietic stem development and in interferon (IFN)-dependent autophagic cell death in T lymphocytes
- First description of in situ cellular dynamics of mycobacterial granulomas in a mammalian host
- Discovery of role of lipoxins in suppressing the cellular response to M. tuberculosis, thereby promoting infection
- Elucidation of opposing roles of IL-1 and interferons in host resistance to M. tuberculosis and the development of an experimental host directed therapy for TB based on the manipulation of these cytokines with eicosanoids
- Development of a murine model for studying the pathogenesis of immune reconstitution inflammatory syndrome (IRIS)
- Discovery of a role of glucocorticoids in the self-regulation of effector CD4+ T-cell function in toxoplasma infection
- Use of hemoxygenase-1 as a clinical biomarker for active TB and its identification as a potential target for host directed therapy
- Discovery of a role for ferroptosis as a mechanism of necrotic cell death in experimental M. tuberculosis infection and its inhibition as a strategy for host directed therapy of TB

