Systems Genomics Section
Denis Voronin, Ph.D.
Staff Scientist

Major Areas of Research
- Interactions between intracellular bacteria and their hosts in Wolbachia-host symbiotic systems
- Host-parasite interactions in filarial worm infections
- Study of arbovirus transmission using vector (mosquito) cells
- Role of microRNA in prokaryotic-eukaryotic cellular interactions
Program Description
Human lymphatic filariasis and onchocerciasis are two of the most debilitating, yet neglected, infectious diseases. Combined, they afflict 150 million of “the poorest of the poor” worldwide. Lymphatic filariasis (elephantiasis) is the second leading cause of morbidity, and onchocerciasis (river blindness) the second leading cause of infectious blindness. Wuchereria bancrofti and Brugia malayi are the causative agents of lymphatic filariasis and Onchocerca volvulus causes onchocerciasis. Filarial parasites have evolved a mutualistic association with the endosymbiotic bacteria, Wolbachia, an association that supports both organisms—neither can survive without the other. Wolbachia are crucial for parasite development, fertility, and viability. It has been postulated that the mechanism(s) underlying the obligate interdependence between Wolbachia and filarial parasites relies on the ability of the bacteria and the parasite to share essential metabolites and biosynthetic processes (glycolysis, nucleotide biosynthesis, iron metabolism). These essential roles make Wolbachia a very attractive drug target for anti-filarial drug development. Moreover, investigating the gram-negative bacteria-eukaryotic cells symbiotic interactions may enable the translation of our study outcomes to better understand bacterial infections in mammalian systems.
Regulation of B. malayi genes through manipulation of worm microRNAs by Wolbachia
Most of what we know about Wolbachia derives from molecular studies performed in insect systems as 70% of arthropods are parasitized by various Wolbachia strains, and a number of these strains can be cultured in insect cells. This is not the case for the Wolbachia of filarial worms. Noticeably, a recent study showed that to survive in the insect host, pathogenic Wolbachia (wMelPop) manipulate host microRNAs (miRNAs) to control arthropod gene expression. We therefore theorize that although the Wolbachia of filarial worms are mutualistic and not pathogenic as in insects, they could also manipulate host miRNAs to control worm gene expression.
Our working hypothesis is that the homeostasis of the mutualistic relationship between parasites and Wolbachia requires the coordinated regulation of parasite genes through Wolbachia-controlled filarial nematode miRNAs. In this project, we focus on Wolbachia-controlled miRNAs and their filarial gene targets and validate the involvement of a specific subset of B. malayi genes in the mutualistic Wolbachia-Brugia association. Any druggable targets could then be tested in vitro and in vivo for their ability to affect the fitness of both Wolbachia and the worm.
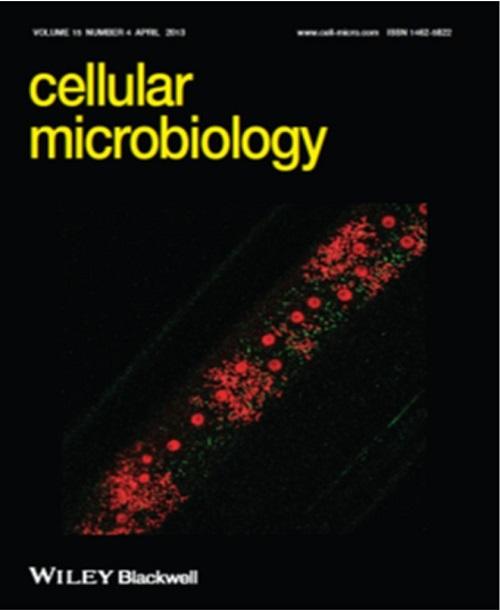
Association of autophagy membrane protein (ATG8a , green) and Wolbachia (small red dots) in the hypodermal cord cells of the filarial nematode Brugia malayi (large red structures are nematode nuclei). Activation and recognition of Wolbachia by autophagy occurs during the periods of rapid bacterial expansion and growth. Image taken using 63× objective. Image appears on the cover of the April 2013 issue of Cellular Microbiology.
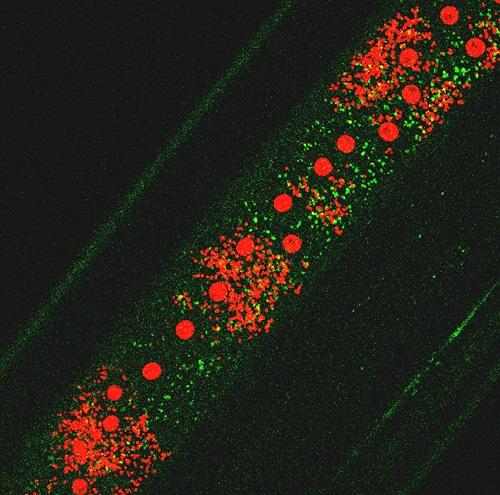
Wolbachia induce Autophagy in Brugia malayi. Green: autophagosomal protein; small red dots: Wolbachia; red spots: worms nuclei.
Activation of autophagy and the elimination of Wolbachia by filarial parasites
Currently, only treatment with antibiotics (doxycycline) that kill the endosymbiont has been shown to have macrofilaricidal effects, i.e. can kill adult worms. Unfortunately, both the dose of doxycycline and the treatment time-course needed to be effective in humans are too high and long. Moreover, the antibiotic cannot be administered to children below the age of 8 and to pregnant women. Since doxycycline is a broad-spectrum antibiotic, widespread use could also promote the development of antibiotic resistant strains of other human pathogenic bacteria. In this project we explore a novel approach for the elimination of Wolbachia: a host(parasite)-oriented treatment that induces the parasite’s innate defense and forces the filarial nematodes to eliminate their endosymbiont. Autophagy is a mechanism used by eukaryotic cells to maintain a healthy intracellular environment and protects them against intracellular invaders (bacteria, virus). We have already shown that Wolbachia load is dependent on the activity of autophagy in insect cells and in filarial nematodes. We are studying the mechanism by which the bacteria manipulate the host’s intracellular defense to protect themselves and, in parallel, we investigate the ways by which the parasite controls the bacterial population to avoid host fitness cost. The discovery of any vulnerable pathways will support a novel strategy that leverages a parasite-oriented treatment to eliminate Wolbachia from the filarial worms and, consequently, affect the survival of the adult parasites.
Biography
Education
Ph.D., Institute of Cytology and Genetics, Novosibirsk, Russia
M.S., Biology, Novosibirsk State University, Russia
Languages Spoken
RussianDr. Denis Voronin obtained his Ph.D. in 2005 from the Institute of Cytology and Genetics in Novosibirsk, Russia. He was then recruited as a postdoctoral researcher for a multi-laboratory project “EndoSymArt” supported by Centre national de la recherche scientifique (CNRS) at the University of Lyon I, France. In 2009, Denis joined the Anti-Wolbachia International Consortium, headed by Professor Mark Taylor, as a senior postdoctoral researcher at the Liverpool School of Tropical Medicine where he worked on alternative approaches to eliminate Wolbachia, an essential endosymbiont of filarial nematodes. In collaboration with Dr. Taylor, he obtained a Bill and Melinda Gates Foundation Grand Challenges Explorations grant to use Brugia malayi’s own intracellular defense to kill the endosymbiont. The project encouraged the use of alternatives for new anti-filarial treatment strategies. In 2014 he joined the New York Blood Center where he started the Cellular Microbiology Research Program. In May 2020, Dr. Voronin joined NIAID’s Laboratory of Parasitic Diseases, Systems Genomics Section as a Staff Scientist.
Selected Publications
Tai W, He L, Zhang X, Pu J, Voronin D, Jiang S, Zhou Y, Du L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020 Jun; 17(6): 613–620.
Grote A, Li Y, Liu C, Voronin D, Geber A, Lustigman S, Unnasch TR, Welch L, Ghedin E. Prediction pipeline for discovery of regulatory motifs associated with Brugia malayi molting. PLoS Negl Trop Dis. 2020 June 23;14(6):e0008275.
Voronin D*, Schnall E, Grote A, Jawahar S, Ali W, Unnasch TR, Ghedin E, Lustigman S. Pyruvate produced by Brugia spp. via glycolysis is essential for maintaining the mutualistic association between the parasite and its endosymbiont, Wolbachia. PLoS Pathog. 2019 Sep;15(9):e1008085. * – Corresponding author.
Tai W*, Voronin D*, Chen J, Bao W, Kessler DA, Shaz B, Jiang S, Yazdanbakhsh K, Du L. Transfusion-Transmitted Zika Virus Infection in Pregnant Mice Leads to Broad Tissue Tropism With Severe Placental Damage and Fetal Demise. Front Microbiol. 2019;10:29. * – Equal contribution
Grote A, Voronin D, Ding T, Twaddle A, Unnasch TR, Lustigman S, Ghedin E. Defining Brugia malayi and Wolbachia symbiosis by stage-specific dual RNA-seq. PLoS Negl Trop Dis. 2017 Mar;11(3):e0005357.
Voronin D, Cook DA, Steven A, Taylor MJ. Autophagy regulates Wolbachia populations across diverse symbiotic associations. Proc Natl Acad Sci U S A. 2012 Jun 19;109(25):E1638-46.
Editorial Experience
- Reviewer: Journal of Parasites and Vectors. Journal of International Parasitology, PLoS Pathogens, PLoS Genetics, PLoS NTDs, PLoS One
- Review Editor: Frontiers in Cellular and Infection Microbiology; Parasite and Host
- Member of Editorial Board: Trends in Vector Research and Parasitology

