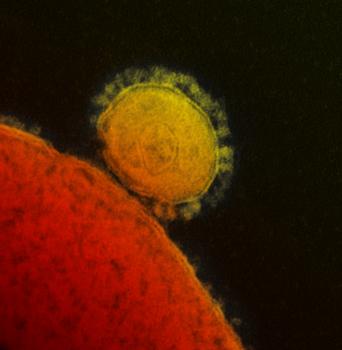
MERS-CoV emerged in 2012, and over 1,300 cases have been reported, with a 30 percent fatality rate (Milne-Price et. al., Path Dis 2014).
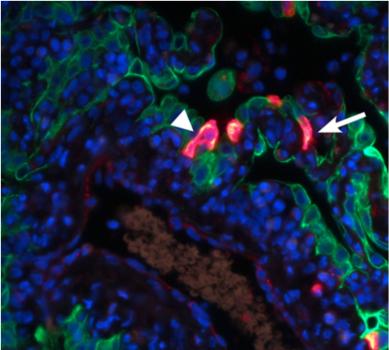
The red staining visualizes the replication of MERS-CoV in the alveoli, the arrowheads indicate type I pneumocytes, and the arrows indicate type II pneumocytes (de Wit et. al., Proc Natl Acad Sci U S A 2013).
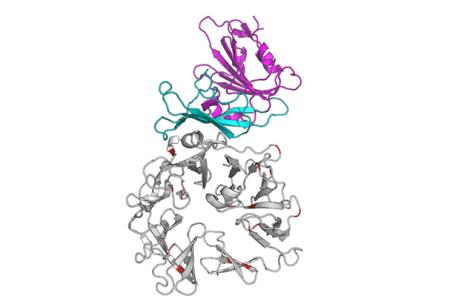
Graphic representation of the interaction between the MERS-CoV spike glycoprotein (blue-purple) and its receptor dipeptidyl peptidase 4 (DPP4). DPP4 is a major determinant of the host tropism of MERS-CoV (van Doremalen et. al., J Virol 2014).

Dromedary camels were identified as the reservoir for MERS-CoV. MERS-CoV appears to have been circulating in dromedary camels over 20 years. (Alagaili et. al., mBio 2014).
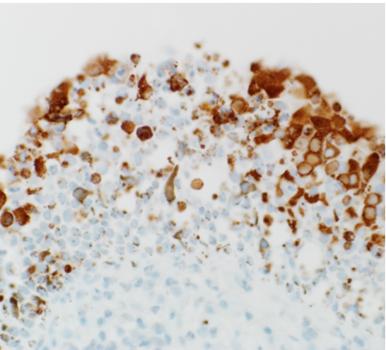
Replication of MERS-CoV in the cells lining the inside of the nose of dromedary camels. MERS-CoV replicates predominantly in the upper respiratory tract of dromedary camels. MERS-CoV is visible as the brown-red staining (Adney et. al., Emerg Infect Dis 2014)

Biosafety level-2 (BSL-2) laboratory of the Virus Ecology Unit. From left to right: postbac Kerri Miazgowicz, Dr. Neeltje van Doremalen, and postbac Shauna Milne-Price.
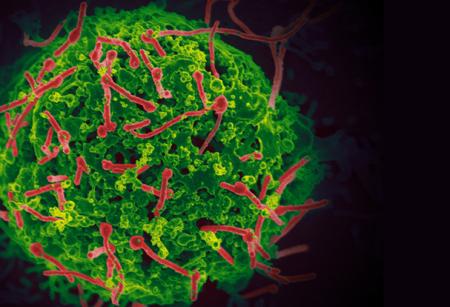
Ebola virus (red) budding from the surface of an infected cell.
Scanning electron micrograph of Ebola virus (red) isolated from the outbreak in West Africa, budding from the surface of a cell. The Ebola virus circulating in humans in West Africa is undergoing relatively few mutations, none of which suggest that it is becoming more severe or transmissible. The study compared sequencing data from samples taken from patients in Guinea (March 2014), Sierra Leone (June 2014), and Mali (November 2014). (Hoenen et. al., Science 2015).

Male hammer-headed fruit bat (Hypsignathus monstrosus) in the Republic of the Congo. Hammer-headed fruit bats are followed over time to study the ecology of Ebola virus.
Obtaining a blood sample from a hammer-headed fruit bat for longitudinal Ebola virus ecology studies. The Virus Ecology Unit embarks on multiple field trips a year to the field site in the Republic of Congo together with collaborators from the Wildlife Conservation Society.
In biosafety level-4 (BSL-4) laboratories, scientists study some of the world's most dangerous viruses. These labs are designed to prevent viruses from being released into the environment and to provide maximum safety for the scientists. In the picture, summer student Jacob Ball and postbac Seth Judson take a tour with Dr. Vincent Munster through RML’s BSL-4 training facility to experience what it is like to work in a BSL-4 laboratory.

The Virus Ecology Unit working together with CDC-Kenya to provide Ebola virus diagnostics during the Ebola virus epidemic in West Africa at the combined CDC/NIH field laboratory in Monrovia, Liberia. From left to right: Dr. Bob Fischer, Jallah Mulbah, Trent Bushmaker, Melvin Ochieng, Dr. Bonventure Juma, and Dr.Vincent Munster. The CDC/NIH field laboratory has analyzed over 5,000 patient samples for presence of the virus during the Ebola virus outbreak.

Dr. Vincent Munster working with blood samples of patients suspected of Ebola virus infection during the Ebola virus epidemic in West Africa 2014 – 2015 . Blood samples are inactivated and analyzed for the presence of the genetic material of Ebola virus.

Sample drop of at the at the combined CDC/NIH field laboratory in Monrovia. The post-mortem samples were collected by the burial teams to determine whether the deceased person died of Ebola. Post-mortem transmission plays a big role in Ebola virus epidemiology. To determine how long Ebola virus could remain infectious in a body after death we designed an experiment where we sampled deceased Ebola-infected monkeys and discovered the virus remained viable for at least seven days. We initially designed this study focused at animals found dead in the wild, but shifted the timing and emphasis to human implications related to the ongoing West Africa Ebola outbreak (Prescott et al, Emerging Infectious Diseases 2015)

