Mucosal Immunology and Virology Unit (MIVU)
Established in 2024
Neeltje van Doremalen, Ph.D.
Chief, Mucosal Immunology and Virology Unit (MIVU)
Stadtman Tenure-Track Investigator
Contact: neeltje.vandoremalen@nih.gov

Major Areas of Research
- Understanding mucosal immunity induced by respiratory virus infections and mucosal vaccination
- Identifying correlates of protection against respiratory virus infections
- Utilize this knowledge to design improved vaccines
Program Description
Our lab is dedicated to understanding the unique role of the mucosal immune system in protecting the respiratory tract against viral infections. Unlike the systemic immune system, the mucosal immune system acts as the first line of defense at critical surfaces such as the respiratory tract, gut, and reproductive organs. Key players in this defense include tissue-resident memory T cells and secretory IgA, which operate independently of systemic responses. We aim to unravel how mucosal immunity is induced and how it provides protection against respiratory viruses, particularly in the context of infections in the upper and lower respiratory tracts.
We investigate the immune responses elicited by respiratory viruses such as influenza A viruses and coronaviruses, focusing on both mucosal and systemic adaptive immunity. Using rodent models, we study the humoral and cellular responses elicited by different infection routes and analyze the role of innate immunity in shaping adaptive responses. By leveraging techniques like high dimensional flow cytometry, systems serology, deep mutational scanning, single-cell transcriptomics, and multiplex imaging, we gain spatial and temporal insights into immune responses across critical tissues, including the nasal-associated lymphoid tissue, nasal turbinates, lungs, and lymph nodes. These studies enable us to map mucosal immunity comprehensively and identify the breadth and depth required for protection upon rechallenge.
We additionally aim to identify correlates of protection and optimize vaccine strategies to induce robust mucosal immunity. We evaluate diverse vaccine platforms—including mRNA, vectored, and subunit vaccines—administered via various routes, such as intranasal, intramuscular, and inhalation. By comparing vaccine technologies and regimens, we aim to establish principles for designing universal vaccines capable of inducing broad, durable mucosal immune responses. Ultimately, our goal is to provide foundational insights that improve vaccine design and our understanding of protective mechanisms against respiratory viruses.
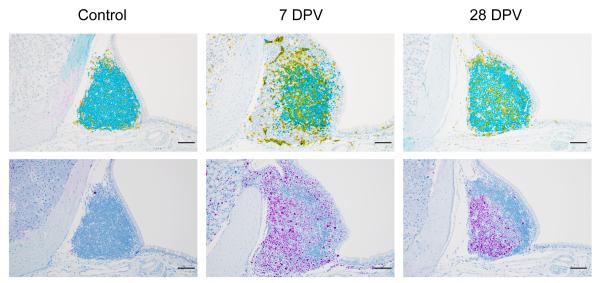
Immunohistochemistry staining of nasal-associated lymphoid tissues (NALT). The upper panels show CD3 (yellow, marking T cells) and PAX5 (teal, marking B cells). The lower panels depict Ki67 (purple, marking proliferating cells). Samples were collected at various time points following intranasal vaccination of mice with a replication-incompetent adenovirus vaccine. Acknowledgements: Reshma K. Mukesh, Carl Shaia, Jessy Prado-Smith.
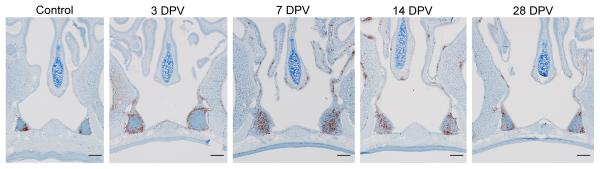
Immunohistochemistry staining of nasal turbinates and NALT tissues with CD3 (brown), highlighting the migration of T cells into these regions. Samples were collected at various time points after intranasal vaccination of mice with a replication-incompetent adenovirus vaccine. Acknowledgements: Reshma K. Mukesh, Carl Shaia, Jessy Prado-Smith.
Biography
Education
Ph.D., Virology, 2013, Imperial College London, United Kingdom
Languages Spoken
DutchDr. van Doremalen was born in the Netherlands and earned her Ph.D. in virology from Imperial College London, where she studied the cellular tropism and transmission of the influenza A virus. She subsequently joined the Laboratory of Virology at the NIAID Rocky Mountain Laboratories in Montana, focusing on emerging infectious diseases in high-containment settings. Her research centered on the ecology and countermeasures for Middle East respiratory syndrome coronavirus (MERS-CoV) and Nipah virus.
When SARS-CoV-2 emerged in late 2019, Dr. van Doremalen leveraged her expertise in MERS-CoV vaccine research to rapidly generate preclinical data for the Oxford-AstraZeneca COVID-19 vaccine, contributing to its global distribution and lifesaving impact. She joined NIAID as a Stadtman tenure-track investigator in 2024.
Currently, Dr. van Doremalen’s research focuses on uncovering the mechanisms of mucosal immune responses in the respiratory tract and understanding how these responses contribute to protection against respiratory viruses.
Selected Publications
Cohen AA, van Doremalen N, Greaney AJ, Andersen H, Sharma A, Starr TN, Keeffe JR, Fan C, Schulz JE, Gnanapragasam PNP, Kakutani LM, West AP Jr, Saturday G, Lee YE, Gao H, Jette CA, Lewis MG, Tan TK, Townsend AR, Bloom JD, Munster VJ, Bjorkman PJ. Mosaic RBD nanoparticles protect against challenge by diverse sarbecoviruses in animal models. Science. 2022 Aug 5;377(6606):eabq0839.
van Doremalen N, Purushotham JN, Schulz JE, Holbrook MG, Bushmaker T, Carmody A, Port JR, Yinda CK, Okumura A, Saturday G, Amanat F, Krammer F, Hanley PW, Smith BJ, Lovaglio J, Anzick SL, Barbian K, Martens C, Gilbert SC, Lambe T, Munster VJ. Intranasal ChAdOx1 nCoV-19/AZD1222 vaccination reduces viral shedding after SARS-CoV-2 D614G challenge in preclinical models. Sci Transl Med. 2021 Aug 18;13(607):eabh0755.
Holbrook MG, Anthony SJ, Navarrete-Macias I, Bestebroer T, Munster VJ, van Doremalen N. Updated and Validated Pan-Coronavirus PCR Assay to Detect All Coronavirus Genera. Viruses. 2021 Apr 1;13(4):599.
van Doremalen N, Lambe T, Spencer A, Belij-Rammerstorfer S, Purushotham JN, Port JR, Avanzato VA, Bushmaker T, Flaxman A, Ulaszewska M, Feldmann F, Allen ER, Sharpe H, Schulz J, Holbrook M, Okumura A, Meade-White K, Pérez-Pérez L, Edwards NJ, Wright D, Bissett C, Gilbride C, Williamson BN, Rosenke R, Long D, Ishwarbhai A, Kailath R, Rose L, Morris S, Powers C, Lovaglio J, Hanley PW, Scott D, Saturday G, de Wit E, Gilbert SC, Munster VJ. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020 Oct;586(7830):578-582.
Folegatti PM, Ewer KJ, Aley PK, Angus B, Becker S, Belij-Rammerstorfer S, Bellamy D, Bibi S, Bittaye M, Clutterbuck EA, Dold C, Faust SN, Finn A, Flaxman AL, Hallis B, Heath P, Jenkin D, Lazarus R, Makinson R, Minassian AM, Pollock KM, Ramasamy M, Robinson H, Snape M, Tarrant R, Voysey M, Green C, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ; Oxford COVID Vaccine Trial Group. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020 Aug 15;396(10249):467-478.
van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL, Thornburg NJ, Gerber SI, Lloyd-Smith JO, de Wit E, Munster VJ. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020 Apr 16;382(16):1564-1567.
Visit PubMed for a complete publication listing.

