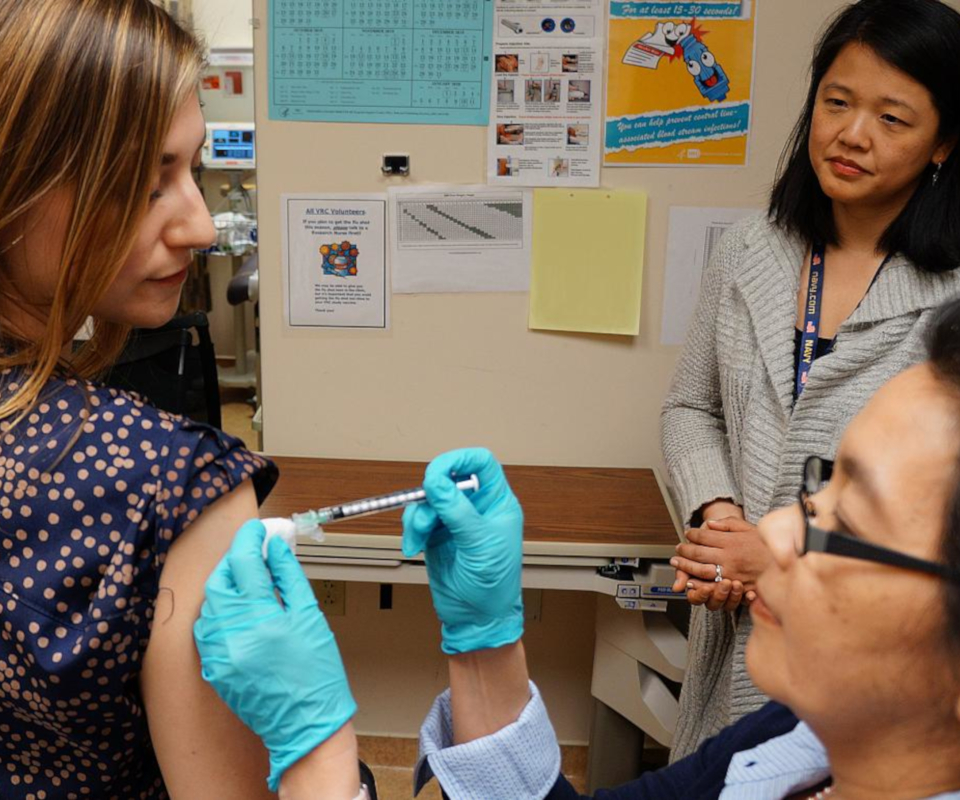
A key focus of NIAID’s influenza research program is developing a universal flu vaccine, or a vaccine that provides robust, long-lasting protection against multiple subtypes of flu, rather than a select few. Such a vaccine would eliminate the need to update and administer the seasonal flu vaccine each year and could provide protection against newly emerging flu strains, potentially including those that could cause a flu pandemic.
Flu viruses are classified by two proteins on the outer surface of the virus: hemagglutinin (H) and neuraminidase (N). There are 18 different H subtypes and 11 different N subtypes, and viruses can be further broken down into different strains within those subtypes. For example, there are various strains of H1N1 influenza virus. The H protein (also called HA) enables the flu virus to enter a human cell. It is made up of a head and a stem. Seasonal flu vaccines fight infection by inducing antibodies that target the HA head. This region varies season to season, which is why flu vaccines must be updated each year. However, scientists discovered the stem typically remains unchanged, making it an ideal target for antibodies induced by a universal flu vaccine.
NIAID Universal Influenza Vaccine Strategic Plan
In February 2018, NIAID released its Universal Influenza Vaccine Strategic Plan outlining the institute’s research priorities. It focuses on three specific research areas to simultaneously broaden knowledge around basic influenza immunity and advance translational research efforts to drive universal influenza vaccine development by:
- Improving knowledge of transmission, natural history and pathogenesis of influenza infection to help understand factors that contribute to the spread, severity and diversity of influenza, and to identify measures to improve disease control.
- Characterizing influenza immunity and immune correlates of protection through the study of immune responses to natural influenza infection and vaccination over time that can be used to identify measurable immune factors critical to vaccine design.
- Supporting rational design of universal influenza vaccines through the development and iterative clinical testing of immunogens, adjuvants, diverse platforms, and alternative vaccine delivery methods. NIAID will continue to build resources (Box 1 below) to lay the foundation of knowledge needed to answer fundamental questions in these research areas and to test new products. These resources will provide the building blocks critical to attaining the research area objectives outlined in the plan with an ultimate goal of rationally designing next-generation influenza vaccines.
Achieving these goals will require a global collaborative effort among government agencies, industry, philanthropic organizations, and academia that incorporates interdisciplinary approaches and new technological tools, such as gene-based vaccination, to aid in the development of vaccine candidates.
Leading Vaccine Strategies and Candidates
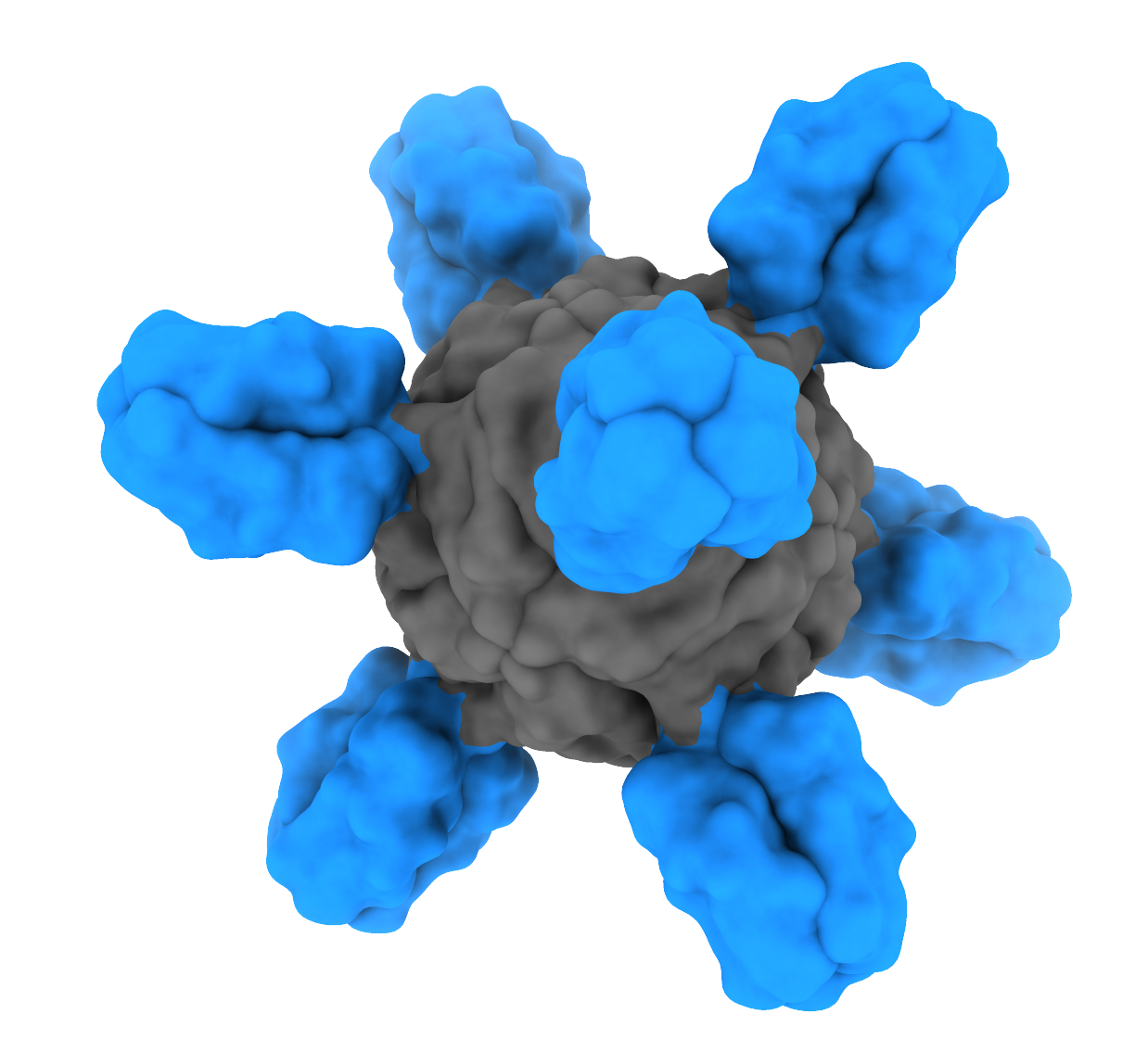
NIAID is studying various strategies to create a vaccine that elicits antibodies targeting the HA stem. For example, NIAID scientists designed an experimental vaccine featuring the protein ferritin, which can self-assemble into microscopic pieces called nanoparticles, as a key component. The vaccine showed promise in animal testing and is being evaluated for future trials in humans.
In another approach to a universal flu vaccine, NIAID scientists developed a vaccine incorporating four subtypes of the H protein into one vaccine. The vaccine is made from non-infectious virus-like particles that stimulate an immune response but cannot replicate or cause disease. Results have been promising in animal studies and may advance to human trials.
NIAID Vaccine Research Center scientists have initiated Phase 1/2 studies of a universal flu vaccine strategy that includes an investigational DNA-based vaccine (called a DNA “prime”) followed by a licensed seasonal influenza vaccine (“boost”) to improve the potency and durability of seasonal influenza vaccines.
In May 2018, NIAID launched a Phase 2 clinical trial of a universal influenza vaccine called M-001. The vaccine, which was developed and produced by BiondVax Pharmaceuticals based in Ness Ziona, Israel, contains antigenic peptide sequences shared among many different influenza strains.
Scientific Advances
NIH Clinical Trial of Universal Flu Vaccine Candidate Begins
September 15, 2023Enrollment in a Phase 1 trial of a new investigational universal influenza vaccine candidate has begun at the National Institutes of Health’s Clinical Center in Bethesda, Maryland. The trial is sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the NIH, and will evaluate the investigational vaccine for safety and its ability to elicit an immune response.
Clinical Trial of mRNA Universal Influenza Vaccine Candidate Begins
May 15, 2023A clinical trial of an experimental universal influenza vaccine developed by researchers at the National Institute of Allergy and Infectious Diseases’ (NIAID) Vaccine Research Center (VRC), part of the National Institutes of Health, has begun enrolling volunteers at Duke University in Durham, North Carolina. This Phase 1 trial will test the experimental vaccine, known as H1ssF-3928 mRNA-LNP,…
Universal Influenza Candidate Vaccine Performs Well in Phase 1 Trial
April 20, 2023Developing a universal influenza vaccine is a significant priority for NIAID scientists. Two new studies describe a unique candidate developed by NIAID's Vaccine Research Center that performed well in a Phase 1 clinical trial.
Trial of Potential Universal Flu Vaccine Opens at NIH Clinical Center
June 28, 2022A Phase 1 clinical trial of a novel influenza vaccine has begun inoculating healthy adult volunteers at the National Institutes of Health Clinical Center in Bethesda, Maryland. The placebo-controlled trial will test the safety of a candidate vaccine, BPL-1357, and its ability to prompt immune responses.
NIH Launches Clinical Trial of Universal Influenza Vaccine Candidate
June 1, 2021A first-in-human, Phase 1 trial assessing the safety and immunogenicity of an investigational nanoparticle influenza vaccine designed to provide long-lasting protection against multiple flu virus strains has begun at the National Institutes of Health Clinical Center in Bethesda, Maryland. Healthy participants 18 to 50 years old will receive either a licensed seasonal influenza vaccine or the…

Tiny Nanoparticles Could Be A Big Jump for Flu Vaccines
May 6, 2021Scientists at NIAID’s Vaccine Research Center and their NIAID-supported colleagues at the University of Washington School of Medicine used tiny nanoparticles to make a huge leap towards the goal of a universal flu vaccine.
NIAID Begins Universal Flu Vaccine Study
April 3, 2019A clinical trial of an innovative universal influenza vaccine candidate known as H1ssF_3928 was developed by scientists at NIAID’s Vaccine Research Center.