Data sharing is a crucial part of responding to infectious disease outbreaks. Timely access to comprehensive data enables researchers and policymakers to take action to mitigate the threat posed by emerging infectious diseases.
As part of NIAID’s public health mission, the institute supports research programs, repositories, and resources that generate and share data and develops policies that facilitate data coordination and sharing to support infectious disease response and countermeasures.
Gathering and sharing data has been a key part of the response to the highly pathogenic avian influenza (HPAI) outbreak. HPAI is caused by the H5N1 virus. Since 2003, H5N1 influenza viruses have circulated in 23 countries, primarily affecting wild birds and poultry. In that time there have been nearly 900 reported human cases, primarily among people who have had close contact with infected birds.
The recent HPAI H5N1 outbreak has spread from birds to infect more than 50 animal species. In March 2024, an HPAI H5N1 outbreak was reported among dairy cows in Texas. As of October 28, H5N1 has been reported in dairy cow populations in 14 states, and 34 human cases have been reported in the United States during 2024. So far, the virus has not shown genetic evidence of acquiring the ability to spread from person to person.
Public health officials from NIAID, the National Institutes of Health (NIH), the U.S. Department of Agriculture (USDA), and the Centers for Disease Control and Prevention (CDC) are closely monitoring the outbreak as part of overarching pandemic preparedness efforts.
Setting the agenda for H5N1 research
In May, NIAID released an H5N1 Influenza research agenda outlining a strategy to understand H5N1 biology and objectives to advance detection, treatment, and prevention. The agenda mobilized various NIAID programs to provide scientific and data support, including:
- Vaccine Research Center (VRC)
- Bacterial and Viral Bioinformatics Resource Center (BV-BRC)
- Centers for Excellence for Influenza Research and Response (CEIRR) Network
- Collaborative Influenza Vaccine Innovation Centers (CIVICs)
The research agenda highlights the importance of studies to understand how H5N1 virus is spreading within and between farms. The scientific community is generating phylogenetic trees using the most recent viral isolates available in GenBank, the publicly available NIH database of annotated genetic sequences. Investigators are also leveraging open-source tools, such as Nextstrain, for real-time analysis of H5N1 sequences to monitor for potential changes in pathogenesis and compatibility to vaccines, therapeutics, and diagnostics.
Best practices for sharing H5N1 data
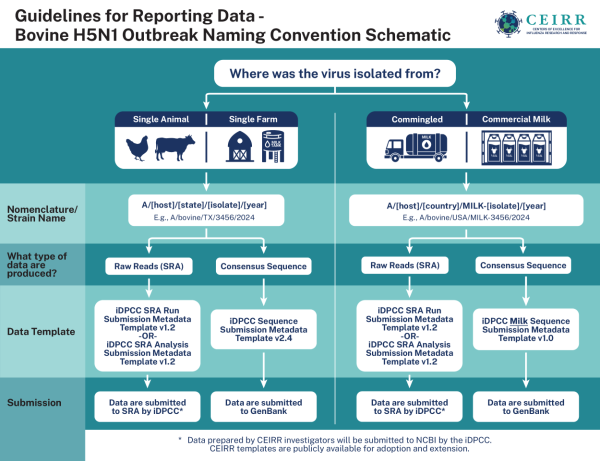
Guidelines for sharing data from the bovine H5N1 outbreak through the CEIRR network.
To support clear and consistent data sharing in the ongoing bovine H5N1 outbreak, Centers of Excellence for Influenza Research and Response (CEIRR) prepared a set of best practice guidelines for the appropriate collection and sharing of data from viral isolate samples related to the outbreak. CEIRR is an NIAID-funded research network that studies the natural history, transmission, and pathogenesis of influenza and provides research infrastructure to address influenza outbreaks.
The best practices for collecting and sharing data from retail milk and other associated HPAI H5 samples include using a centralized repository, applying standardized metadata templates and consistent H5N1 sequence nomenclature, and rapidly reporting sensitive results.
The CEIRR best practices align with NIAID and NIH data sharing policies, leverage public data repositories, and are tailored to meet the needs of an evolving public health concern. They also establish the data sharing infrastructure for identifying and containing H5N1 in the event that it spreads from human to human.
“The CEIRR program created these guidelines because the sampling of retail food products for evidence of viral contamination is not something that has traditionally occurred in the influenza field," explained Dr. Erik Stemmy, Ph.D., CEIRR team lead and NIAID Respiratory Diseases Branch Deputy Chief. "Because there was not an established process to report this information and share it, we wanted to ensure that the CEIRR investigators were standardized and recommend that other researchers use this format as much as possible so that data can be shared and interpreted more easily.”
Learn more about how NIAID is advancing H5N1 research: