Contact
Submit a Media Request
Contact the NIAID News & Science Writing Branch.


Submit a Media Request
Contact the NIAID News & Science Writing Branch.
Submit a Media Request
Contact the NIAID News & Science Writing Branch.
Submit a Media Request
Contact the NIAID News & Science Writing Branch.
Visit AccessClinicalData@NIAID to review and request access to NIAID Clinical Trials data.
Allow the research community access to clinical data sets to harness the power of data to generate new knowledge to understand, treat, and prevent infectious diseases such as COVID-19.
Contact accessclinicaldatasupport@niaid.nih.gov for questions or help.
NIAID provides guidance on data-management and -sharing practices to ensure NIAID’s research adheres to NIH policies to serve knowledge sharing, secondary use, and reproducibility of NIAID-funded research data as well as enable opportunities to develop a data science workforce.
Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19, New England Journal of Medicine, December 11, 2020
Remdesivir for the Treatment of Covid-19 — Final Report, New England Journal of Medicine, November 5, 2020
NIAID Clinical Trials Data Repository, AccessClinicalData@NIAID, is an NIAID cloud-based, secure data platform that enables sharing of and access to reports and data sets from NIAID COVID-19 and other sponsored clinical trials for the basic and clinical research community.
Biomedical and health data coupled with powerful advanced data analytical and statistical tools provide innovative opportunities to accelerate the development of new and improved therapeutic interventions and diagnostics, improved prevention strategies and disease surveillance, and implement new and improved design of clinical trials.
This NIAID research resource provides a foundation with potential to improve the health of people in the United States and around the world.
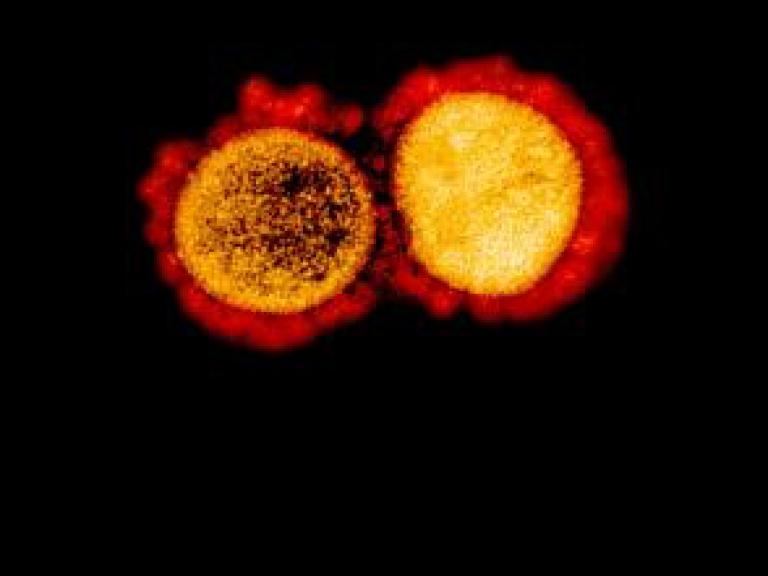
Accessclinicaldata@NIAID has a new data set available from ACTIV-1 (Accelerating COVID-19 Therapeutics, Interventions and Vaccines) Clinical Trial sponsored by the U.S. Department of Health and Human Services Biomedical Advanced Research and Development Authority and the National Center for Advancing Translational Sciences, National Institutes of Health.
The global network involves multidisciplinary investigations into how and where viruses and other pathogens can emerge from wildlife and spillover to cause disease in people. Research is led by 10 Centers and one Coordinating Center and will involve collaborations with peer institutions in the United States and 28 other countries.
Read more about this network: CREID Network
Research projects will include surveillance studies to identify previously unknown viral causes of febrile illnesses in humans, the animal sources of viral or other disease-causing pathogens, and to determine what genetic or other changes make these pathogens capable of infecting humans. Other research by the CREID investigators will involve developing reagents and diagnostic assays to improve detection of emerging pathogens as well as studies aimed at detailing human immune responses to new or emerging infectious agents. Overall, the breadth of research projects to be carried out in the CREID network will allow for study of disease spillover in multiple phases of the process: where pathogens first emerge from an animal host; at the borders between wild and more populated areas, where and when rapid human-to-human transmission occurs; and, finally, how transmission is facilitated in urban areas, where epidemic spread can occur.
Primary awardees for the CREID network and regions of focus include:
Submit a Media Request
Contact the NIAID News & Science Writing Branch.
Submit a Media Request
Contact the NIAID News & Science Writing Branch.
TATFAR was created in 2009 to address the urgent threat of antimicrobial resistance (AMR). TATFAR’s technical experts from Canada, the European Union (EU), Norway, the United Kingdom, and the United States collaborate and share best practices to strengthen domestic and global efforts to combat AMR.
The Centers for Disease Control and Prevention (CDC) currently serves as the secretariat for TATFAR, providing administrative support and maintaining the website for the taskforce.
U.S. representatives to TATFAR include the U.S. Department of Health and Human Services (co-chair), NIH (NIAID), CDC, the Food and Drug Administration, the Biomedical Advanced Research and Development Authority, the U.S. Department of Agriculture, the U.S. Department of Defense, and the Environmental Protection Agency.
Read more about this network: TATFAR
TATFAR’s goal is to improve cooperation between the United States and EU in four key areas:
The Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X) global partnership was created to help address the threat of antibiotic resistance.
CARB-X is funded by the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response (ASPR) in the United States Department of Health and Human Services; the Wellcome Trust in the United Kingdom; Germany’s Federal Ministry of Education and Research (BMBF); the United Kingdom Government’s Department of Health and Social Care (DHSC), through its Global Antimicrobial Resistance Innovation Fund (GAMRIF); the Bill & Melinda Gates Foundation; and the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH). For more information, please see Funding Partners.
Read more about this network: CARB-X
CARB-X is focused on the preclinical discovery and development and Phase 1 clinical trials of new antibacterial products (including therapeutics, vaccines, and diagnostics) to help address the threat of antibiotic resistance.
Over the last several decades there has been a continual withdrawal of pharmaceutical companies engaged in developing new antibiotics. In 1990, there were at least 18 large pharmaceutical companies actively developing antibiotics. As of April 2020, there are 4.
CARB-X provides funding for companies with innovative and promising solutions to antibiotic resistance. CARB-X also provides the business support and drug development expertise to that companies, including start-ups, need to increase their odds of success. NIAID provides technical support and preclinical drug development services to CARB-X awardees.
Anita Sheoran, Ph.D., Office of Biodefense, Research Resources and Translational Research