Universal Influenza Candidate Vaccine Performs Well in Phase 1 Trial
mRNA Version of NIAID Vaccine Begins Similar Testing
Scientists at NIAID’s Vaccine Research Center (VRC) report in two new studies that an experimental influenza vaccine, designed to elicit immunity against a broad range of influenza viruses, performed well in a small trial of volunteers. In fact, the vaccine has advanced to a second trial led by scientists at Duke University through NIAID’s Collaborative Influenza Vaccine Innovation Centers (CIVICs).
In a phase 1 clinical trial of 52 volunteers, the vaccine developed by the VRC – known as H1ssF (influenza H1 hemagglutinin stabilized stem ferritin nanoparticle vaccine) – was safe, well-tolerated, and induced broad antibody responses that target the hemagglutinin stem. The two new studies assessing the nanoparticle vaccine published April 19 in Science Translational Medicine.
Healthy volunteers ages 18-70 enrolled at the NIH’s Clinical Center and were given either a single 20-microgram dose or two 60-microgram vaccine doses. Boosters were given 16 weeks after the initial dose. The trial enrolled between April 1, 2019, and March 9, 2020.
Trial participants did not experience any severe adverse events; the most common vaccine reactions included mild headache, tenderness at the vaccine site, and temporary general discomfort.
As anticipated based on preclinical study results, H1ssF generated binding antibodies to the stem of the influenza H1 hemagglutinin (HA) protein. Antibody responses were observed regardless of dose or participant age. “These responses were durable, with neutralizing antibodies observed over one year after vaccination,” the authors stated, suggesting this vaccine prototype can advance further universal influenza vaccine development.
The H1ssF vaccine using an mRNA delivery system also began testing in a phase 1 clinical trial being overseen by scientists at the Duke Human Vaccine Institute, a part of NIAID’s CIVICs network.
HA is composed of head and stem domains and enables the influenza virus to attach and enter a human cell. The immune system can mount an immune response to HA, but most of the response is directed toward the head. Influenza vaccines must be updated each year because the HA head constantly changes – a phenomenon called “antigenic drift.” The new vaccine candidate consists only of the HA stem. The stem is more conserved than the head between influenza strains and subtypes, and thus is less likely to change every season. Scientists predict that targeting the HA stem without the distraction of the HA head could induce stronger and longer-lasting immunity.
Influenza A viral HA can be divided into groups 1 and 2, and further subdivided into multiple subtypes based on their sequences. Scientists used the stem of an HA from a group 1 influenza virus to create the nanoparticle vaccine. Both group 1 and group 2 influenza viruses are among those responsible for seasonal influenza as well as the sporadic and deadly outbreaks of avian influenza viruses with pandemic potential. The H1ssF vaccine elicited responses that broadly neutralized group 1 influenza A viruses. Additional clinical trials are underway to test a ferritin nanoparticle-based vaccine designed to elicit group 2 influenza A viruses, and to test the H1ssF and the group 2 vaccine together in a cocktail aimed at approaching universal influenza vaccine coverage.
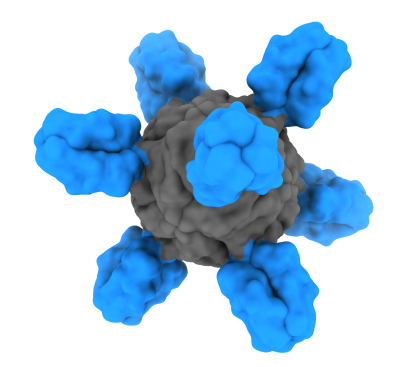
The H1ssF vaccine is unique in that it only displays the stem part of the influenza HA protein on the surface of a nanoparticle made of nonhuman ferritin. Ferritin spontaneously self-assembles into an eight-sided nanoparticle. When designed to display a part of the HA protein, the ferritin-HA proteins form particles displaying HA spikes on their surface, mimicking the natural organization of HA on the influenza virus. Displaying influenza HA surface proteins on the outside of the nanoparticle makes them easily accessible to immune cells that encounter the nanoparticle. The immune system can then learn to develop antibodies against displayed proteins.
References:
A Widge, et al. An Influenza Hemagglutinin Stem Nanoparticle 1 Vaccine Induces Cross
Group 1 Neutralizing Antibodies in Healthy Adults. Science Translational Medicine DOI: 10.1126/scitranslmed.ade4790 (2023).
S Andrews, et al. An Influenza H1 Hemagglutinin Stem-Only Immunogen Elicits a Broadly Cross-Reactive B Cell Response in Humans. Science Translational Medicine DOI: 10.1126/scitranslmed.ade4976 (2023).
ClinicalTrials.gov search identifier NCT03814720.