Prion diseases are transmissible, untreatable, and fatal brain diseases of mammals. Their cause is highly unusual: The host’s normal prion protein can, for unknown reasons, malfunction and assemble into structured aggregates called prions that cause infectious brain disease. This process – which can be underway for years before symptoms appear – likely causes the most common form of prion disease in people, sporadic Creutzfeldt-Jakob disease (CJD). Other forms of human prion diseases include variant CJD, fatal familial insomnia, Gerstmann-Straussler-Scheinker Syndrome and Kuru.
In livestock and wildlife, prion diseases such as scrapie (sheep), chronic wasting disease (deer, elk, moose), and mad cow disease (cattle) can spread by casual contact or contamination of feeds or the environment. Prions also can be infectious if inadvertently transferred from person to person by invasive medical procedures.
Prion disease symptoms reflect the brain being destroyed and can range from memory loss and unstable movement to being unable to sleep or realize the need to eat.
NIAID scientists have focused research on prion structures, biochemistry, cell biology, pathogenesis, diagnostics, and therapeutics. NIAID also is exploring similarities between prion diseases and other protein misfolding diseases, such as Alzheimer’s and Parkinson’s diseases, Lewy body dementia, and chronic traumatic encephalopathy.
Highlights
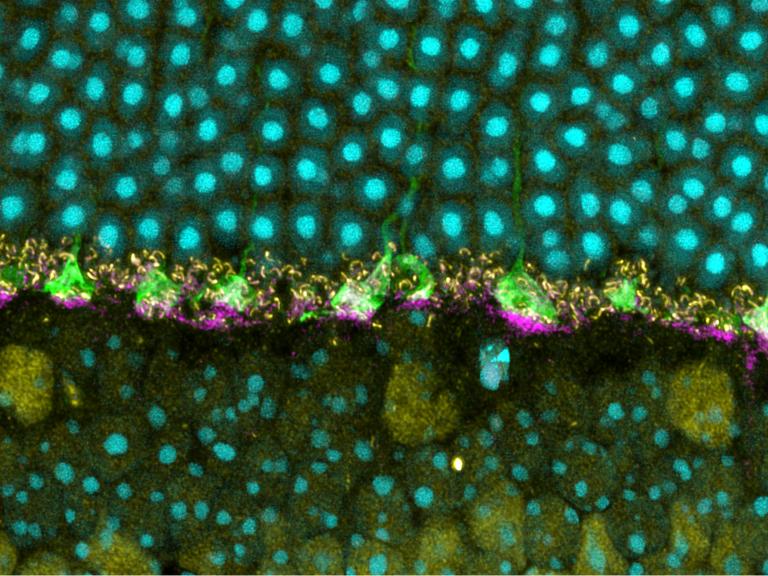
The Eyes Have it: A Functional Role for Prion Protein
Answers to what a normal prion protein does could help lead them to develop treatments and disease-prevention measures against human prion diseases, such as Creutzfeldt-Jakob disease, fatal familial insomnia, and kuru.
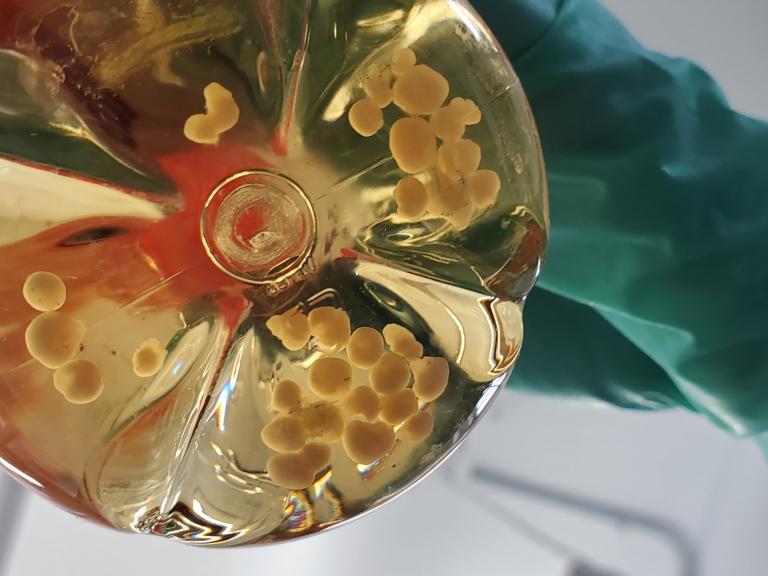
NIH Study Shows Chronic Wasting Disease Unlikely to Move from Animals to People
A new study of prion diseases, using a human cerebral organoid model, suggests there is a substantial species barrier preventing transmission of chronic wasting disease (CWD) from cervids—deer, elk and moose—to people.
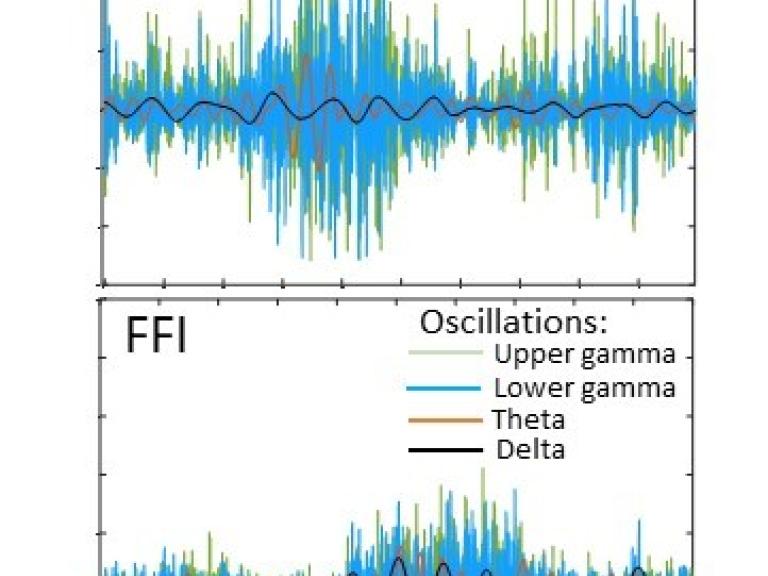
Novel Study Model Reveals New Understanding of Fatal Familial Insomnia
Fatal familial insomnia (FFI) is a little-known yet horrific disease in which people die from lack of sleep. In a new study, NIAID scientists developed a cerebral organoid model to study the exact protein mutation that causes FFI.
NIAID Now Blog
- The Eyes Have it: A Functional Role for Prion Protein
September 26, 2024


