Tissue Analysis Core
Richard Koup, M.D.
Deputy Director, Vaccine Research Center
Chief, Immunology Laboratory and Immunology Section
Acting Chief, Vaccine Immunology Program
Acting Head, Tissue Analysis Core
Contact: rkoup@mail.nih.gov

Major Areas of Research
- Development and application of novel imaging methods for analysis of tissue cells
- T cell dynamics in secondary lymphoid organs in natural infection (HIV/SIV) and after vaccination
- Generating “tissue imaging signatures” in cancer and cancer/HIV
Program Description
The mission of the Tissue Analysis Core is the development, optimization and application of cutting-edge imaging assays for the analysis of human and nonhuman primate (NHP) tissues in support of VRC goals.
Imaging methodologies are a prerequisite for the understanding of:
- the spatial analysis of tissue immune cells and with respect to tissue structure
- the local interactions between immune cells, tissue stromal cells and tissue-expressed antigens (pathogens, cancer, self-antigens)
- the role of local inflammatory mediators for the dynamics of tissue immune cells
A comprehensive tissue analysis requires the application of complementary technologies allowing for the characterization of phenotype, localization, function and molecular signatures of cells under investigation. Merging cutting-edge technologies like multidimensional imaging, multiparameter flow cytometry and deep sequencing allows for the generation of “imaging signatures” that could provide information about viral persistence in specific tissue areas-sanctuaries and assist in the discovery of biomarkers for disease progression and interventional strategies with specific immunotherapies and vaccines.
Current technologies employed in the Tissue Analysis Core include:
multispectral confocal imaging (A),
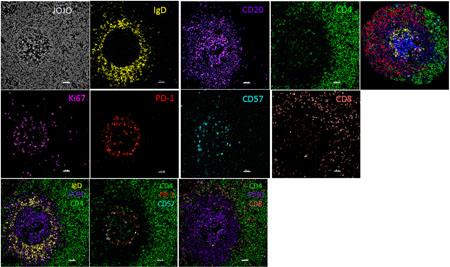
Example of multispectral confocal imaging
Credit: NIAIDRNAscope methodology that allows for the simultaneous detection of mRNA and several protein markers (B),
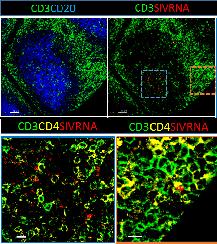
Example of RNAscope methodology
Credit: NIAIDlive imaging and v) TIRF microscopy (C).
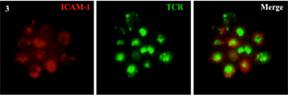
Example of live imaging and TIRF microscopy
Credit: NIAIDTissue Mass Spectrometry Imaging Unit, Bruker rapifleX TissueTyper MALDI-TOF/TOF mass spectrometer
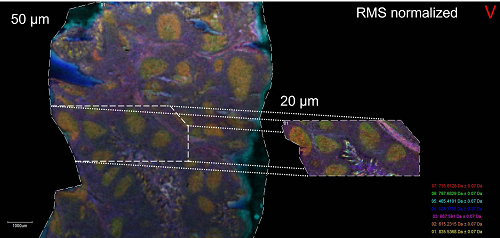
This platform allows for label-free imaging of lipids, metabolites, proteins, and other ionizable chemical species directly from tissue samples in either targeted or untargeted/unsupervised mode.
Credit: NIAID
Several analytical tools (Imaris, Voloom, Histo-cytometry platform and in house developed algorithms) are used for the quantitative analysis of the obtained imaging objects.
Biography
Education
M.D., 1982, Johns Hopkins University School of Medicine, Baltimore, MD
M.S., 1979, University of Connecticut, Stamford, CT
B.S., 1978, University of Connecticut, Stamford, CT
Dr. Koup received his B.S. in biophysics in 1978 and his M.S. in biochemistry in 1979 from the University of Connecticut. He attended Johns Hopkins University School of Medicine, where he obtained his M.D. in 1982. Dr. Koup served an internship and residency in internal medicine with the Rhode Island Hospital, Brown University Medical School, Providence, Rhode Island, from 1982 to 1985. He served in a clinical fellowship (infectious diseases) at the Worcester Memorial Hospital and a research fellowship (viral immunology), at UMass Medical Center, Worcester. Dr. Koup is board certified in both internal medicine and infectious diseases. Dr. Koup previously held several academic appointments at the University of Texas Southwestern Medical Center that include chief, division of infectious disease; professor, internal medicine; professor, microbiology; and the Jay P. Sanford Professor of Infectious Diseases.
Selected Publications
Padhan K, Moysi E, Noto A, Chassiakos A, Ghneim K, Perra MM, Papaioannou V, Fabozzi G, Ambrozak D, Poultsidi A, Loannou M, Fenwick C, Sekaly RP, Pantaleo G, Koup RA, Petrovas C. Acquisition of optimal TFH cell function is defined by specific molecular, positional and TCR dynamic signatures. Proc Natl Acad Sci USA (accepted).
Potter EL, Gideon HP, Tkachev V, Fabozzi G, Chassiakos A, Petrovas C, Darrah PA, Lin PL, Foulds KE, Kean LS, Flynn JL, Roederer M. Measurement of leukocyte trafficking kinetics in macaques by serial intravascular staining. Sci Transl Med. 2021 Jan 13;13(576):eabb4582.
Shankwitz K, Pallikkuth S, Sirupangi T, Kirk Kvistad D, Russel KB, Pahwa R, Gama L, Koup RA, Pan L, Villinger F, Pahwa S, Petrovas C. Compromised steady-state germinal center activity with age in nonhuman primates. Aging Cell. 2020 Feb;19(2):e13087.
Austin JW, Buckner CM, Kardava L, Wang W, Zhang X, Melson VA, Swanson RG, Martins AJ, Zhou JQ, Hoehn KB, Fisk JN, Dimopoulos Y, Chassiakos A, O'Dell S, Smelkinson MG, Seamon CA, Kwan RW, Sneller MC, Pittaluga S, Doria-Rose NA, McDermott A, Li Y, Chun TW, Kleinstein SH, Tsang JS, Petrovas C, Moir S. Overexpression of T-bet in HIV infection is associated with accumulation of B cells outside germinal centers and poor affinity maturation. Sci Transl Med. 2019 Nov 27;11(520):eaax0904.
Ferrando-Martinez S, Moysi E, Pegu A, Andrews S, Nganou Makamdop K, Ambrozak D, McDermott AB, Palesch D, Paiardini M, Pavlakis GN, Brenchley JM, Douek D, Mascola JR, Petrovas C, Koup RA. Accumulation of follicular CD8+ T cells in pathogenic SIV infection. J Clin Invest. 2018 May 1;128(5):2089-2103.
Research Group
Development and implementation of high resolution and high throughput imaging assays for the analysis of tissue immune dynamics in viral infections and vaccine settings.

