The Division of Intramural Research (DIR), a component of the National Institute of Allergy and Infectious Diseases, has long been at the forefront of research on immunologic, allergic, and infectious diseases. For more than 60 years, DIR scientists have discovered new pathogens, deciphered normal immune system function, identified aberrations underlying immunological diseases, and developed FDA-approved vaccines and therapies.
This history of scientific advances tells the stories behind them—the findings, their significance, and the collaborators involved. Each story is a snapshot of how public investment in medical research helps to move science forward and improve health in the United States and abroad.
Today, DIR scientists are advancing knowledge of immune system components and functions, defining mechanisms responsible for abnormal immune functions, increasing understanding of the biology of infectious agents and the host response to infection, and conceiving strategies to prevent and treat infectious and immune-mediated diseases. With our partners in academia and industry, we are making exciting progress in translating lab breakthroughs into new and improved diagnostics, drugs, and vaccines. With continued commitment to cutting-edge medical research, we expect even more game-changing discoveries in the years to come.

Boy in Liberia, West Africa.
Inventing the First Rapid Diagnostic Test for Malaria
Basic research conducted in NIAID labs during the mid-1980s gave rise to ParaSight F, the first rapid diagnostic test (RDT) for malaria. NIAID scientists characterized a unique protein, called Pf HRP-2, found in abundance in the deadliest malaria parasite, Plasmodium falciparum. They then proposed that its presence in the blood could be used to diagnose P. falciparum infection. In 1989, Becton, Dickinson, and Company (BD) licensed this technology and used it to develop ParaSight F.
The test proved that it was possible to make an accurate malaria diagnostic that didn’t require a microscope, electricity, or lengthy training to perform. It was an ideal tool for health care workers in rural malaria-endemic areas. BD began selling ParaSight F in 1995, but the test was not commercially successful, and BD took it off the market in 2000.
Today, more than a decade after ParaSight F provided the prototype, the needs of freshly invigorated malaria control programs have helped expand the market for RDTs. Companies from around the world make diagnostics based on rapid Pf HRP-2 detection similar to ParaSight F. The World Health Organization (WHO) is evaluating malaria RDTs to help guide national malaria control programs. So far, more than 35 commercially available RDTs have met WHO performance criteria.

Left to right: Robert Purcell, M.D.; Albert Kapikian, M.D.; and Stephen Feinstone, M.D., first identified hepatitis A virus (HAV) in 1973. Their groundbreaking work led to development of the first licensed HAV vaccine.
Developing the World’s First Licensed Hepatitis A Vaccine
NIAID scientists played a crucial role in the development of Havrix, the world’s first licensed hepatitis A vaccine. Hepatitis A is an infectious disease of the liver caused by the hepatitis A virus (HAV). In 1973, NIAID researchers first identified HAV in fecal samples from infected human volunteers. In subsequent studies, they isolated and cultivated the HAV strain, called HM175, used to create the vaccine. They also developed methods to weaken and inactivate HM175 so it would trigger immune responses without causing disease, and tested HM175’s protective effect in animal models.
In 1988, SmithKlineBeecham (now called GlaxoSmithKline [GSK]) partnered with NIAID to develop HM175 as a candidate vaccine. GSK researchers conducted large clinical trials to determine its safety and effectiveness. NIAID, the Centers for Disease Control and Prevention (CDC), and the U.S. Army also were significantly involved in development of the vaccine, which received regulatory approval in Europe in 1991 and in the United States in 1995.
Today, Havrix helps prevent HAV infection in military personnel and travelers destined for areas where the virus is common. In the United States, hepatitis A vaccination is recommended for all children over the age of 1 and for high-risk groups such as men who have sex with men. According to the CDC, the rate of new hepatitis A infections in the United States declined by more than 92 percent between 1995 and 2008.

Doctor examining baby.
Developing the World’s First Licensed Hepatitis A Vaccine
Respiratory syncytial virus (RSV) is a leading cause of bronchiolitis and pneumonia in children under 1 year of age. RSV infection can be life-threatening, especially for babies born prematurely or with health problems such as chronic lung disease or congenital heart disease. Fortunately, doctors have a powerful tool against RSV: a preventive treatment that originated in NIAID laboratories and today is manufactured by MedImmune as Synagis.
RSV was first isolated from children more than 50 years ago. In 1985, NIAID researchers showed that giving anti-RSV proteins, or antibodies, to cotton rats protected them from infection. They then developed monoclonal antibodies—antibodies that bind to the same target on an invading microbe—and demonstrated their ability to attach to and neutralize RSV in animals.
MedImmune licensed the monoclonal antibodies from NIAID in 1989, made one of them suitable for human use, and conducted clinical trials showing that monthly administration of the antibody could protect high-risk infants from severe RSV disease.
The Food and Drug Administration approved Synagis in 1998. According to the American Academy of Pediatrics, monthly administration of the drug to high-risk infants during RSV season reduces RSV-related hospitalizations by 45 to 55 percent.
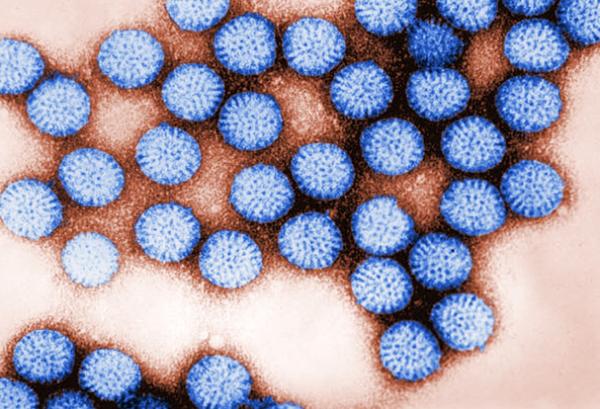
Rotavirus gets its name from its wheel shape (rota is Latin for wheel).
Creating the First Rotavirus Vaccine
Rotavirus is the most common cause of severe childhood diarrhea worldwide, responsible for more than 500,000 deaths each year. In 1974, after NIAID scientists first identified human rotavirus in the United States, they began what would become a decades-long effort to develop a vaccine. The researchers defined the mode of transmission of rotavirus, identified the viral proteins critical for triggering an immune response, and formulated a vaccine aimed at protecting people against several important rotavirus strains.
Their efforts, in partnership with Wyeth-Ayerst Laboratories, led to the development, testing, and Food and Drug Administration approval in 1998 of RotaShield, the first rotavirus vaccine. This success was temporary, however, as post-marketing studies linked RotaShield to an increased risk of bowel obstruction. Though this side effect was rare, the vaccine was voluntarily withdrawn from the market in 1999.
While RotaShield’s risks were considered unacceptable in the United States, many scientists argued that its protective benefits could save thousands of lives in developing countries, where most rotavirus-associated deaths occur. The vaccine has since been licensed to a U.S. nonprofit organization and is undergoing clinical testing in Ghana to evaluate the efficacy in infants of two oral doses: one administered soon after birth and another before the infant is 60 days old.
A second-generation rotavirus vaccine developed by the same NIAID scientists has been licensed by pharmaceutical companies in Brazil, China, and India. These companies can make the vaccine locally at much less expense, raising hopes that children in developing nations will one day have greater access to an affordable rotavirus vaccine.
Nurse administers FluMist vaccine to a patient.
Developing a Needle-Free Flu Vaccine
Decades of NIAID-funded research helped create FluMist, the first nasal spray vaccine for influenza approved by the Food and Drug Administration. The vaccine, manufactured by MedImmune, contains live but weakened influenza viruses that are cold-adapted, meaning that they reproduce only in cooler temperatures found in the nose and upper respiratory tract. Because the viruses cannot replicate at normal body temperature, they do not cause illness, but they do prepare the immune system for any future flu encounters.
Researchers at the University of Michigan established the cold-adapted viruses for use in the vaccine in 1967. During the 1970s, NIAID funded scientists within and outside the Institute to study and further develop the cold-adapted flu strains to the point where they could enter large-scale human testing. In 1995, NIAID partnered with a pharmaceutical company to begin evaluating the vaccine in clinical trials, many of which were conducted by the NIAID-supported Vaccine and Treatment Evaluation Units. FluMist first received Food and Drug Administration approval in 2003.
Today, the vaccine is recommended for healthy, non-pregnant individuals aged 2 to 49 years. It provides a welcome alternative for those who want their seasonal flu shot without the needle. In September 2009, another version of FluMist was approved to protect the same population from 2009 H1N1 influenza.
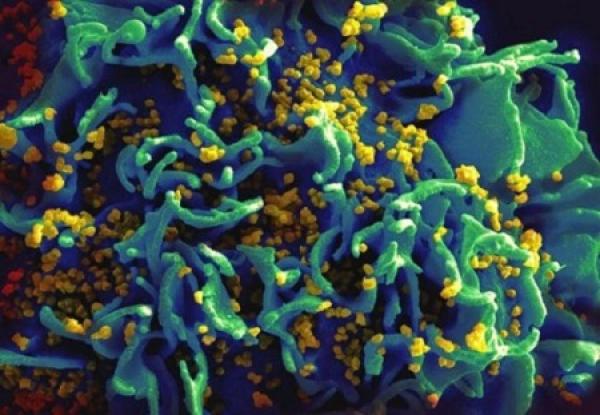
HIV-infected T cell.
Finding HIV’s Hideouts
During the mid-1990s, there was considerable optimism that highly active antiretroviral therapy (HAART), regimens of three or more anti-HIV drugs, could eradicate the virus from infected patients. Researchers had shown that HAART reduced the amount of HIV to often undetectable levels in the blood. A cure seemed closer than ever. This optimism, however, was short-lived.
In late 1997, building on the earlier work of researchers at Johns Hopkins University, three groups of NIAID-funded scientists independently demonstrated that a small pool of HIV-infected T cells persists in people who receive HAART for prolonged periods of time. This latent reservoir of HIV enables the virus to hide from the onslaught of potent drug cocktails and to rebound to high levels in the blood when therapy is stopped.
The discovery showed that HIV was even more formidable than previously thought. It also brought about many new studies on the role, function, mechanisms, and potential vulnerabilities of HIV reservoirs. NIAID continues to lead these efforts by launching new initiatives aimed at advancing basic and clinical studies on HIV persistence and pursuing strategies for identifying and eradicating latent reservoirs.

During the early 1970s, NIAID researcher Anthony S. Fauci, M.D., and colleagues developed an effective treatment regimen for severe vasculitis.
Reversing a Once-Fatal Blood Vessel Disease
During the early 1970s, NIAID scientists developed a life-saving treatment regimen for anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis. In this autoimmune disease, antibodies attack immune cells called neutrophils, causing inflammation in small- to medium-sized blood vessels. This leads to potentially life-threatening organ damage, particularly in the airways, lungs, and kidneys.
Based on NIAID's work, the standard of care for ANCA-associated vasculitis evolved into a two-step approach consisting of a 3- to 6-month course of cyclophosphamide plus steroids to induce remission, followed by long-term daily use of the immune-suppressing drug azathioprine plus steroids. This treatment regimen turned these once-fatal diseases into chronic conditions in which most people can achieve remission. However, long-term immunosuppressive therapy can cause severe side effects, such as cancer and infertility.
In 2010, results from an NIAID-sponsored clinical trial showed that treating ANCA-associated vasculitis with the drug rituximab once a week for only 4 weeks provided the same benefits as six months of standard cyclophosphamide therapy. Based on these results, the Food and Drug Administration in 2011 approved rituximab plus steroids for the treatment of ANCA-associated vasculitis.
In 2013, NIAID-funded researchers reported long-term results from the trial, showing that rituximab is as effective as standard therapy at inducing and maintaining disease remission over 18 months. These findings promise to improve the quality of life for patients and continue NIAID's strong legacy of research against this devastating disease.
Photomicrograph of cells infected with human herpesvirus 6.
Advancing the Prevention and Treatment of Herpesvirus Infections
Eight herpesviruses are known to infect humans. They include herpes simplex virus (HSV) types 1 and 2, which cause oral and genital herpes, and varicella-zoster virus (VZV), which causes chickenpox and shingles. These viruses all cause lifelong infection, although when dormant, they do not cause symptoms.
During the early 1980s, NIAID scientists were among the first to demonstrate that daily oral therapy with the antiviral drug acyclovir reduces the frequency of outbreaks of genital herpes. They also showed that genital herpes can be spread by sexual partners who display no symptoms of HSV infection. This finding helped dispel the notion that only those experiencing an outbreak can transmit the virus.
This research team also cloned and mapped the VZV genome and demonstrated that a strain of VZV that causes chickenpox can later reactivate to cause shingles in the same person. During the late 1990s, NIAID helped design and conduct the Shingles Prevention Study, which proved that an experimental VZV vaccine reduced both the incidence and severity of shingles in older adults.
The Food and Drug Administration approved the shingles vaccine in 2006. Today, the Centers for Disease Control and Prevention recommend the vaccine for all adults aged 50 and older.

Ethiopian girl carries jug of water. Outbreaks of hepatitis E can occur in areas where there is limited access to clean water.
Developing the First Hepatitis E Vaccine
An estimated one-third of the world's population has been infected with hepatitis E virus (HEV). It is a major cause of disease in the developing world, and recent studies have shown that it’s more common in wealthy nations than previously thought.
Humans acquire the virus from contaminated food or water. Symptoms include nausea, vomiting, diarrhea, and jaundice. Most people recover after a few months, but hepatitis E can be deadly: its mortality rate among pregnant women is approximately 20 percent.
In 1980, two independent groups of researchers—one at NIAID and one in India—discovered HEV after analyzing stool samples obtained from different hepatitis outbreaks. Over the next decade, the NIAID researchers developed a candidate HEV vaccine and showed that it could protect animals from several HEV strains.
NIAID then partnered with SmithKline Beecham, now GlaxoSmithKline (GSK), to perform additional testing of the candidate vaccine in animals and, ultimately, in humans. The Walter Reed Army Institute of Research also collaborated on the clinical development of the vaccine. After small-scale clinical trials in the United States and Nepal showed promising results, NIAID, GSK, and the U.S. Army conducted a larger trial in Nepal in collaboration with the Nepalese Army. This trial confirmed that the vaccine was safe and highly effective: three doses were 96 percent effective at preventing hepatitis E; two doses were 87 percent effective.
Despite this success, the hepatitis E vaccine has yet to be licensed for use in developing countries. NIAID continues to seek licensing partners to manufacture and distribute the vaccine in nations where HEV is common.
Discovering How HIV Enters Immune Cells
By the mid-1980s, scientists knew that HIV entered immune cells by attaching to a surface molecule called CD4. They also knew that CD4 alone wasn’t enough to allow HIV to enter and infect cells. There had to be at least one other factor involved.
In May 1996, NIAID researchers solved part of this mystery by discovering a co-receptor, called CXCR4, that enables certain HIV strains to enter their primary target, immune system T cells. Just one month later NIAID researchers struck again, finding another co-receptor, called CCR5, that allows most HIV strains to enter T cells as well as macrophages, another population of immune cells.
These discoveries helped scientists answer additional questions about HIV disease, including why certain people do not become infected with HIV despite repeated exposure. For example, researchers found that some individuals have a mutation in the gene that codes for CCR5, thereby disabling its function and preventing the virus from entering cells. This revelation gave rise to a new class of anti-HIV drugs called CCR5 antagonists, which block cell entry by CCR5-using HIV strains. The first such drug, called maraviroc, was licensed by the Food and Drug Administration in 2007 for use in drug combinations in patients with drug-resistant HIV.
Basic research continues to uncover new leads that may inform development of future AIDS medicines. In 2008, NIAID scientists identified another cellular receptor for HIV called integrin alpha 4 beta 7, a discovery that has opened new areas of investigation into how the virus attacks the immune system in the gut, now known to be a key feature of how HIV disease develops.
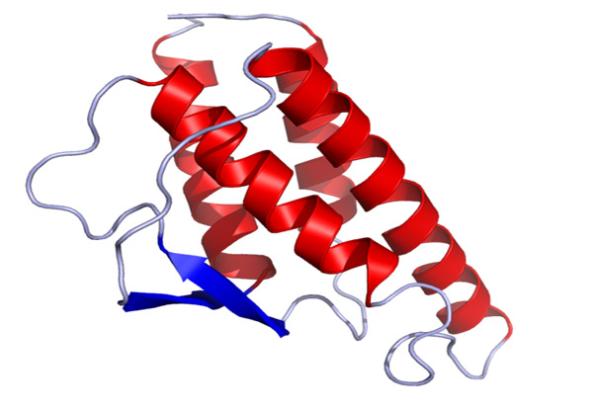
Crystal structure of human IL-4.
Improving the Understanding of How Cells Communicate
Cytokines are molecules that help cells communicate with other cells and tissues. They are involved in a number of biological processes, such as inflammation, immunity, and cell specialization and growth. There are over 100 known cytokines. The major producers of and targets for cytokines are immune cells. Cytokines stimulate immune cells to become active in response to infection and also can prevent the immune response from overwhelming the host.
During the 1980s, NIAID investigator William Paul, M.D., identified and characterized the cytokine interleukin-4 (IL-4) and its receptor. Dr. Paul showed that IL-4 is a central regulator of allergic diseases and is critical for the production of immunoglobulin E (IgE), the allergy antibody. His work also demonstrated that IL-4 directs T cells to become T helper type 2 (Th2) cells that help antibody-producing cells fight bacteria, parasites, and viruses. Blocking IL-4 is being explored as a treatment for diseases caused by IgE and Th2 immune responses, such as asthma and certain cancers.
Chemokines are specialized cytokines that attract white blood cells to sites of infection and inflammation by binding to receptors found on the cell surface. During the early 1990s, NIAID investigator Philip Murphy, M.D., discovered the first chemokine receptors, and showed that they formed a family within the G protein-coupled receptor superfamily, which is a major source of drug targets for a broad range of diseases. Because chemokine receptors are selectively expressed on immune cells, strategies to block them are now being developed to treat a variety of immune-mediated diseases, such as asthma, rheumatoid arthritis, multiple sclerosis, and HIV.

NIAID pox-vector technology has been used in vaccines to protect companion animals from infectious disease.
Protecting Animals from Infectious Diseases
A technology conceived during the 1980s by NIAID researchers is today being used to protect millions of animals from serious infectious diseases. NIAID’s pox-vector technology, licensed in 2006 by animal product company Merial, has been developed into more than 15 different vaccines approved by the U.S. Department of Agriculture to safeguard companion animals, farm animals, and wild animals from diseases that are often fatal, including feline leukemia, rabies, distemper, and avian influenza.
A pox-vectored vaccine uses weakened versions of a poxvirus to deliver modified genetic material from another infectious organism. The vaccine provides just enough viral material to stimulate the immune system, but it does not cause disease in an otherwise healthy animal.
For example, Merial’s Raboral V-RG is an oral pox-vectored vaccine for rabies. It is encased in solid bait, which enables health officials to immunize large numbers of wildlife by airplane or helicopter, or by hand. Local, state, and federal agencies have used oral rabies vaccines such as Raboral in more than 15 states to curtail the geographic expansion of rabies spread by raccoons, the most frequently reported rabid wildlife species.
NIAID pox-vector technology has been used in vaccines to protect companion animals from infectious disease.

Scientist prepares a tissue culture.
Transforming the Study of Viruses, Cancer
During the 1950s, NIAID scientist Harry Eagle, M.D., defined the fundamental compounds that human and other mammalian cells need to grow in laboratory culture, or outside a living organism. This mixture, today known as Eagle’s minimum essential medium or EMEM (pronounced e-mem), is used by scientists around the world in their study of viruses and cancer.
Viruses need living cells to multiply. Before Dr. Eagle’s discovery, researchers typically grew cells using animal blood serum, which contains substances that may kill viruses or inhibit their growth. Dr. Eagle found that a synthetic cocktail of amino acids, salts, vitamins, and glucose provided the basic nutrients required for mammalian cells to multiply. He further found that adding small amounts of animal blood serum to this mixture helped sustain cell growth.
Dr. Eagle’s breakthrough enabled scientists to efficiently grow and maintain viruses in the lab and gain valuable insights into their infectious processes and potential vulnerabilities. It was now easier for cancer researchers to study differences between normal and malignant cells and to evaluate the effects of anti-tumor agents.
Today, many viral vaccines, including those for polio, measles, and chickenpox, are produced using mammalian cell cultures grown in EMEM or its derivatives. The same is true for monoclonal antibodies, lab-produced, infection-fighting proteins used in diagnostics and treatments for some cancers, infectious diseases, and autoimmune disorders.

This 1928 photo shows Building 1 of what would become NIAID Rocky Mountain Laboratories campus in Hamilton, MT.
Identifying Major Causes of Tickborne Disease
During the 20th century, scientists at what is now NIAID’s Rocky Mountain Laboratories (RML) in Hamilton, MT, identified three bacterial causes of infectious disease. These seminal discoveries led to those organisms bearing the scientists’ names—one of the highest honors a microbiologist can achieve.
In 1909, Dr. Howard T. Ricketts described his isolation of the tickborne bacterium that causes Rocky Mountain spotted fever. The pathogen was later named Rickettsia rickettsii in recognition of his efforts. Dr. Ricketts did much of his work near the site of the present-day RML.
In 1940, RML researcher Dr. Herald R. Cox reported that his group had isolated a Rickettsia-like bacterium transmitted from a tick in Montana. The agent was found to be identical to a bacterium described by Australian researcher Frank M. Burnet and said to be the cause of query fever, also known as Q fever. Both Cox and Burnet were credited with identifying the infectious agent, which today is named Coxiella burnetii.
RML researchers reported another major discovery in 1982, when Dr. Willy Burgdorfer identified the bacterium that causes Lyme disease. That agent is now known as Borrelia burgdorferi. Dr. Burgdorfer’s discovery came while he was examining deer ticks from the northeastern United States. He noticed that most had a spiral-shaped bacterium called a spirochete. Additional experiments confirmed that the spirochete was the Lyme disease bacterium.
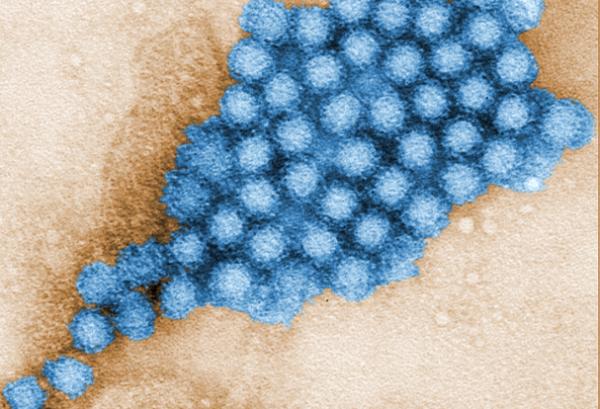
Transmission electron micrograph of norovirus particles.
Helping Detect and Control Norovirus Outbreaks
Noroviruses are infamous for wreaking havoc on cruise ships and at day care centers, where they can easily be transmitted by infected people or contaminated surfaces, water, or food. Worldwide, they are the most common cause of acute gastroenteritis, an illness that can lead to abdominal cramps, diarrhea, and vomiting in all age groups. While there is no vaccine to prevent norovirus infection, early outbreak detection and control can be facilitated by a test based in part on technology developed in NIAID laboratories.
Human noroviruses cannot yet be grown in the laboratory, making it difficult to study their interaction with immune cells. NIAID researchers sidestepped this challenge by modifying the DNA of baculoviruses to carry the gene that encodes the protein shell, or capsid, of several norovirus strains. When grown in insect cells, the modified baculoviruses express virus-like particles (VLPs) that mimic the norovirus capsid, which contains immune-stimulating antigens.
In 2004, diagnostics manufacturer R-Biopharm licensed NIAID’s recombinant baculoviruses and used the virus-derived VLPs to create monoclonal antibodies against norovirus. These antibodies are used in the company’s Ridascreen Norovirus 3rd Generation EIA test to capture norovirus antigen from a stool sample. If antigen is present, the antibodies bind to it so it can be detected in later steps of the test. According to R-Biopharm, the test yields results in less than 2 hours, enabling faster implementation of outbreak control procedures. The Food and Drug Administration approved the test in 2011.
NIAID’s contribution to the Ridascreen test adds to its legacy of achievement in norovirus research. In 1972, NIAID scientists were the first to identify norovirus, initially called Norwalk virus, as a cause of acute epidemic gastroenteritis.

Prion protein, shown in red, can become infectious and cause neurodegenerative disease. Here four nerve cells in a mouse illustrate how infectious prion protein moves within cells along neurites – wire-like connections the nerve cells use for communicating with adjacent cells.
Advancing Rapid Detection of Prion Diseases in Animals and Humans
Prion diseases, such as Creutzfeldt-Jacob disease (CJD) in humans, scrapie in sheep, and mad cow disease in cattle, destroy the brain and damage other organs. The diseases are difficult to diagnose, untreatable, and ultimately fatal. People and animals can be infected for years before symptoms appear.
NIAID scientists are developing a blood test to diagnose prion diseases in people and animals. A blood test would likely be faster and more practical than present diagnostic tests that use cerebral spinal fluid or brain tissue.
The latest in a series of blood-test advances by NIAID researchers is called enhanced quaking-induced protein conversion (eQuIC). This method uses an antibody to isolate abnormal prion protein from blood plasma and an amplification reaction to enhance detection. The test is 10,000 times more sensitive for detecting variant CJD than previously described tests, and accurately differentiated between healthy hamsters and those infected with scrapie. NIAID and its project partner, Swiss diagnostics manufacturer Prionics AG, have applied for a patent on the eQuIC test.
eQuIC could be used by blood banks, hospitals, livestock operations, and rendering plants to screen for prion diseases. The test concept also could potentially extend to the diagnosis of other diseases characterized by abnormal proteins, such as Alzheimer’s, Huntington’s, and Parkinson’s, but much more research is needed.
Four nerve cells in a mouse illustrate how infectious prion protein, in red, moves within cells along neurites—wire-like connections the nerve cells use to communicate with adjacent cells.

Robert M. Chanock, M.D. (left), and Robert J. Heubner, M.D., in the NIAID Laboratory of Infectious Diseases. Dr. Chanock is perhaps best known for discovering human respiratory syncytial virus and the four pararinfluenza viruses. Dr. Heubner and colleagues were the first to isolate human adenovirus.
Discovering Causes of Respiratory Disease
NIAID laboratories have a long and distinguished history of identifying previously unknown respiratory pathogens. In 1953, NIAID scientists were the first to isolate human adenovirus. The researchers later worked with industry partners to develop a highly effective oral vaccine against adenovirus 4, which would go on to receive Food and Drug Administration (FDA) approval. The vaccine has been used off and on for decades to help protect U.S. military recruits from adenovirus infection, commonly referred to as “boot camp flu.”
NIAID researchers also identified and characterized human respiratory syncytial virus (RSV), the most common cause of serious lower respiratory tract disease in infants and children worldwide. The investigators subsequently developed and helped bring to FDA licensure an antibody to prevent RSV disease in high-risk infants. Several RSV vaccine candidates are in various stages of development in NIAID labs.
Other respiratory agents isolated by NIAID scientists include the four parainfluenza viruses (important causes of childhood respiratory disease), new strains of rhinovirus and coronavirus (causes of the common cold), and Mycoplasma pneumoniae (a cause of bacterial pneumonia). After the isolation and characterization of M. pneumoniae, NIAID researchers went on to show that pneumonia caused by the bacterium could be treated effectively with the antibiotic tetracycline.
LAD2 mast cells.
Understanding Inflammation One Cell at a Time
The mast cell is a bone marrow-derived cell that is most notable for producing histamine, a compound that triggers the inflammatory immune response. In healthy people, mast cells defend against disease-causing microbes. But when mast cells release histamine and other mediators in response to harmless substances, such as pollen and food proteins, allergic reactions such as asthma, and even life-threatening anaphylaxis, may result.
One of the barriers to understanding how normal mast cells function is the costly and time-consuming methods needed to isolate mast cells from human tissues. These methods also yield only a small number of cells. In 2003, NIAID investigators in the Laboratory of Allergic Diseases developed a human mast cell line called LAD2, which closely resembles normal mast cells. By studying LAD2 cells in the lab, investigators have been able to characterize and better understand how mast cells function and respond to various stimuli. Researchers also have used LAD2 cells to identify molecular causes for allergies and to develop novel therapies to treat allergic diseases, such as asthma, and inflammatory diseases, such as rheumatoid arthritis.
Since their development, LAD2 cells have been supplied to over 200 academic institutions, licensed worldwide by more than 30 biotech and pharmaceutical companies, and used in more than 60 published studies. The cell line has become a powerful tool to advance research in allergic and inflammatory diseases.
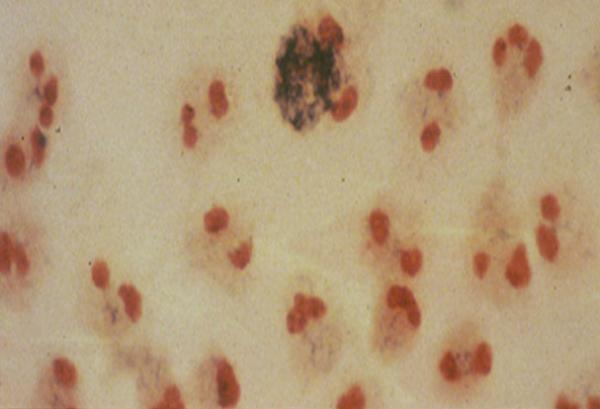
Neutrophils from the blood of a patient with CGD.
Identifying and Understanding Rare Immune System Diseases
Primary immune deficiency diseases (PIDDs) are rare disorders caused by inherited defects in cells of the immune system. People with PIDDs generally are more susceptible to infections and may have other medical issues, including autoimmune diseases, weakened lung function, and tumors.
NIAID investigators and their colleagues have made significant contributions to the current understanding of the causes of PIDDs and how to treat patients affected by these devastating diseases. Notably, scientists at NIAID and the National Human Genome Research Institute identified autoimmune lymphoproliferative syndrome, or ALPs, a disorder characterized by the abnormal accumulation of white blood cells in the lymph nodes, liver and spleen, leading to anemia, hemorrhage, and increased risk of bacterial infections. NIAID investigators also discovered the mutation that causes hyper-immunoglobulin E syndrome, or Job’s Syndrome, a disorder that makes people susceptible to recurrent boils on the skin and lungs.
Since the 1980s, NIAID investigators have examined the causes of and developed new treatments for chronic granulomatous disease (CGD), a syndrome that makes people susceptible to bacterial and fungal infections; severe combined immunodeficiency, a syndrome that results in a complete lack of B- and T-cell function, causing severe, life-threatening viral infections; and immunodeficiency caused by mutations in the NEMO gene, which lead to frequent bacterial and viral infections and abnormal teeth, hair, skin, and nails. NIAID investigators also discovered DOCK8 immunodeficiency, a disease characterized by persistent skin infections, allergies, and cancer, and XMEN disease, which is characterized by persistent Epstein-Barr virus infections.
In 2007, NIAID started a Primary Immune Deficiency Clinic that accepts all patients with known or suspected PIDDs and offers them treatment recommendations and, in some cases, a disease diagnosis.
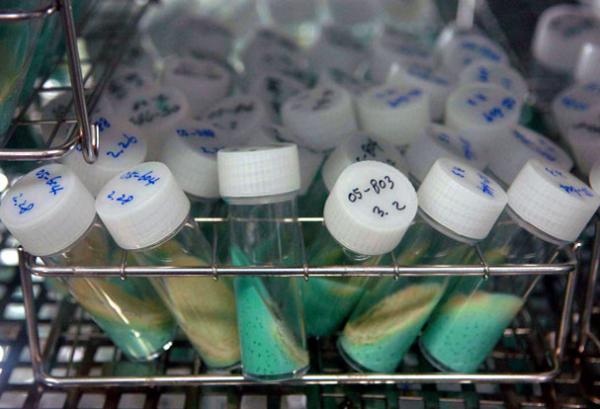
Sputum cultures from tuberculosis patients. Sputum often is used to detect and identify bacteria in the lungs.
Developing Novel Tuberculosis Drugs
Current tuberculosis (TB) treatment regimens require people to take several antibiotic drugs for at least six months. The difficulty of faithfully completing these treatments has contributed to the emergence of drug-resistant strains of Mycobacterium tuberculosis (Mtb), the bacterium that causes the disease. NIAID supports and conducts research to identify new and improved TB drugs, and scientists have uncovered numerous drug candidates during the past two decades. In the late 1990s, NIAID researchers tested more than 60,000 compounds related to the TB drug ethambutol. They identified several compounds that were effective against Mtb in the laboratory and collaborated with the pharmaceutical company Sequella on further drug development. Mouse studies revealed that a compound called SQ109 kills Mtb, including drug-resistant strains, in the animals’ lungs. Early human clinical studies indicated that SQ109 is safe, and additional clinical trials are underway to evaluate SQ109’s effectiveness. The Food and Drug Administration (FDA) has granted SQ109 Orphan Drug and Fast Track status, which could help accelerate eventual FDA approval. In 2000, NIAID scientists and collaborators reported the discovery of another potential TB drug, PA-824. In the laboratory, the drug eliminates both actively growing Mtb and a dormant form of the bacterium, suggesting that it might be effective against latent TB infections. Currently, there are no drugs that specifically target latent TB. PA-824 entered clinical trials in 2005, and initial results suggest that it is safe and effective. NIAID researchers and NIAID-funded scientists also are investigating the anti-TB action of existing drugs. For example, in 2012, results from an NIAID-funded clinical trial indicated that the antibiotic linezolid is effective against extensively drug-resistant TB.
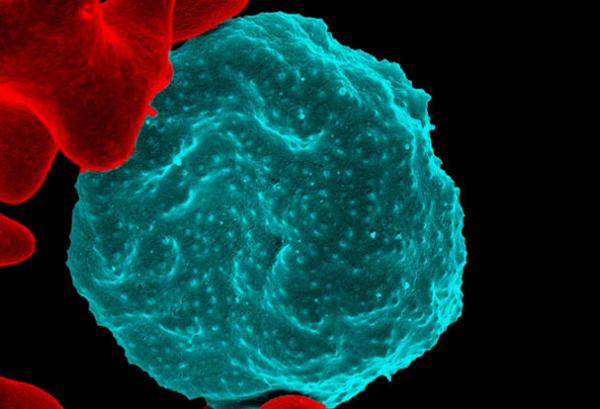
Colorized scanning electron micrograph of red blood cell infected with malaria parasites, which are colorized in blue. The infected cell is in the center of the image area. To the left are uninfected cells with a smooth red surface.
Understanding Human Resistance to Malaria
NIAID researchers have played a crucial role in determining what makes some people resistant to malaria. Understanding malaria resistance mechanisms promises to help scientists identify ways to mimic these protective effects using drugs or vaccines.
During the mid-1970s, NIAID researchers found that people who lack a red blood cell protein called the Duffy antigen are resistant to Plasmodium vivax, the most geographically widespread of the five malaria parasites that infect humans. NIAID scientists were the first to show that P. vivax depends on the Duffy antigen for entry into red blood cells (RBCs).
More recently, NIAID research has shed light on other mechanisms of malaria resistance. Certain parasite proteins alter the surfaces of infected RBCs, causing them to stick to blood vessels. NIAID scientists found that RBCs from malaria-infected children with a type of oxygen-carrying hemoglobin called hemoglobin C have lower amounts of a specific parasite protein. This trait impaired the cells’ stickiness and may make African children less prone to deadly cerebral malaria, which is caused by parasite-infected RBCs accumulating in the brain.
A separate NIAID study in Mali, Africa, showed that a deficiency in the enzyme glucose-6-phosphate dehydrogenase confers protection against severe, life-threatening malaria. Continued study of these and other human resistance mechanisms will help scientists understand how malaria parasites cause disease and develop new ways to control malaria.
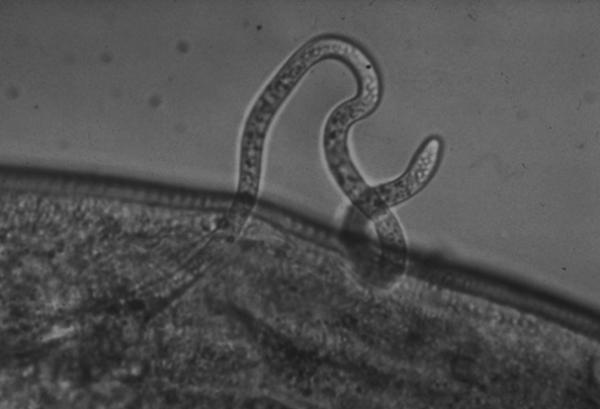
An O. volvulus parasite emerges from an infected blackfly.
Advancing Early, Rapid Diagnosis of River Blindness
The eye and skin infection known as onchocerciasis, or river blindness, affects more than 18 million people worldwide, mostly in rural African communities near streams and rivers. The disease is caused by the parasite Onchocerca volvulus, which is spread through the bite of an infected blackfly. Symptoms of onchocerciasis, which can include severe itching and eye lesions, develop slowly and may not become apparent until years after infection.
Currently, health care workers diagnose onchocerciasis by removing small pieces of skin to look for O. volvulus larvae. However, this technique cannot detect early infections. The lack of a quick and inexpensive test to detect O. volvulus makes it difficult to track new infections and provide timely treatment.
During the early 1990s, NIAID scientists identified Ov16, an O. volvulus protein that is abundant in the early stages of infection. The researchers showed that antibodies against Ov16 can be detected in the blood of infected people up to one year before infection appears in the skin. This simple blood test showed promising results in initial field trials conducted in seven West African villages during late 1999 and early 2000.
In 2013, NIAID investigators licensed the technology to the Program for Appropriate Technology in Health for the development of a rapid, noninvasive, and inexpensive diagnostic test that health care workers in resource-poor settings can use. By enabling early detection and treatment of river blindness, this test promises to aid efforts to eradicate the disease.

Cryptococcus neoformans
Understanding and Treating Fungal Infections
Fungal infections have emerged as a growing health threat, especially in people whose immune systems have been weakened by HIV or other causes. Cryptococcosis, caused by the ubiquitous fungus Cryptococcus neoformans, rarely affects people with healthy immune systems but is the most common fungal disease in HIV-infected people. If untreated, it can lead to fatal brain infection.
During the 1950s, NIAID researcher Chester Emmons, Ph.D., discovered the environmental source of C. neoformans. The fungus is found throughout the world in soil contaminated with bird excrement. Although only the asexual, yeast-like form of the fungus has been found in infected humans, C. neoformans also can reproduce sexually. Sexual reproduction of C. neoformans, which was first described by NIAID scientist K.J. Kwon-Chung, Ph.D., during the 1970s, forms infectious spores.
In addition to advancing knowledge of C. neoformans biology, NIAID scientists have focused on developing treatments for fungal infections. Much of the early work on the antifungal drug amphotericin B was performed at NIAID. Although effective at treating cryptococcosis, the drug can cause serious side effects, such as kidney damage. In 1979, NIAID scientists found that a combination of amphotericin B and flucytosine is as effective as and less toxic than high-dose amphotericin B.
Nearly two decades later, NIAID-funded scientists reported that amphotericin B plus flucytosine, followed by the antifungal drug fluconazole, substantially decreases the risk of dying from cryptococcosis in patients with AIDS or other immunodeficiencies. Today, this is the standard regimen for cryptococcosis treatment in HIV-positive people and other immunocompromised patients. NIAID continues to conduct and fund studies of potential new treatments for cryptococcosis and other fungal diseases.

Dr. Fairhurst’s Cambodian colleague approaches a villager’s home to assess a patient with malaria.
Understanding and Detecting Drug-Resistant Malaria
Since the 1940s, highly effective malaria drugs have reduced deaths from this mosquito-borne parasitic disease. The drug chloroquine was widely used after World War II, but Plasmodium falciparum, the most deadly malaria parasite, quickly developed ways to evade the drug’s effects. Chloroquine-resistant parasites emerged in Southeast Asia in the 1950s and spread into Africa over the next two decades.
In 2000, NIAID scientists discovered that mutations in a single parasite gene cause chloroquine resistance. In collaboration with colleagues in the United States and Mali, Africa, the NIAID researchers developed a molecular marker that can be used to diagnose people infected with chloroquine-resistant malaria parasites and to investigate the origin and worldwide spread of these parasites.
Today, control of P. falciparum malaria mainly depends on artemisinin-based combination therapy, which combines fast-acting artemisinin with longer-lasting drugs. However, parasites resistant to artemisinin are beginning to emerge and spread in Southeast Asia. A better understanding of the molecular mechanism of artemisinin resistance could help researchers design new antimalarial treatments and potentially prolong the usefulness of existing drugs.
In 2013, NIAID scientists, together with French and Cambodian colleagues, developed a new test that detects artemisinin-resistant parasites in malaria-infected people. This test will enable health officials to track the spread of resistance and choose appropriate treatments, as well as help scientists screen new malaria drug candidates in the laboratory.

