Molecular Immunoengineering Section
Masaru Kanekiyo, D.V.M., Ph.D.
Chief, Molecular Immunoengineering Section
Stadtman Investigator
Contact: kanekiyom@mail.nih.gov

Highlight
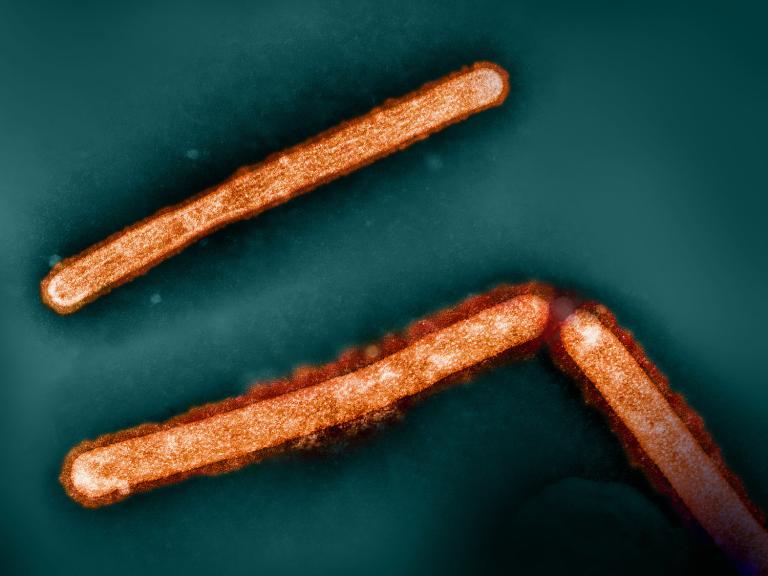
Single Dose of Broadly Neutralizing Antibody Protects Macaques from H5N1 Influenza
February 11, 2025
A single dose of a broadly neutralizing antibody given prior to virus exposure protects macaques from severe H5N1 avian influenza, NIH scientists report.
Major Areas of Research
- Vaccine design and immunoengineering
- Antigen display and delivery systems
- Immune profiling and antibody discovery
- Assay and animal model development
Program Description
The Molecular Immunoengineering Section (MIS) at the Vaccine Research Center aims to conceive novel vaccine concepts that elicit broad and potent protective immune responses against influenza virus and provide a mechanistic principle for designing vaccines for other hypervariable pathogens such as coronaviruses and HIV-1. MIS is dedicated to advancing vaccine immunogen design beyond structure-based protein engineering by combining concepts and principles from multiple disciplines, including, but not limited to, immunobiology, biochemistry, biophysics, nanotechnology, and computational biology. The mission of the MIS is to define the fundamental rules behind vaccine-elicited immunity. Our interests span from basic immunology and virology to translational sciences with the goal of advancing vaccine concepts that would radically improve immune responses to vaccines. Our work involves various biochemical, biophysical, structural, immunological, and computational techniques and tools. MIS efforts include development of “supraseasonal” and “pre-pandemic” influenza vaccine candidates, “mosaic” antigen display technology, high-throughput high-definition virus neutralization assay systems, and animal models that recapitulate key aspects of human responses to influenza, such as immunological imprinting, preexisting immunity, antibody specificity and repertoire, immunodominance, and pathogenesis. MIS is also integrated with the VRC’s Influenza Program by serving as the lead of the Vaccine Concepts team. The Influenza Program involves multiple VRC Sections and Programs with the goal of advancing candidate vaccines from bench to clinic.
Biography
Education
D.V.M., 2002, Nihon University
Ph.D., 2006, Nihon University
Dr. Masaru Kanekiyo obtained his D.V.M. in 2002 and Ph.D. in 2006. Dr. Kanekiyo did his postdoctoral training at the Vaccine Research Center (VRC) with Dr. Gary Nabel studying protein engineering and nanoparticle-based vaccine design. In 2014, Dr. Kanekiyo joined the laboratory of Dr. Barney Graham at the VRC as a Staff Scientist and was later appointed head of the Molecular Immunoengineering Unit. In 2022, Dr. Kanekiyo was named Chief of the Molecular Immunoengineering Section (MIS) at the VRC. Dr. Kanekiyo is also an Earl Stadtman Tenure-Track Investigator. Dr. Kanekiyo focuses his efforts on understanding the vaccine-host interface to define immunological principles that inform the design of effective vaccines against highly challenging targets, such as influenza virus. His interests include vaccine design, protein engineering, self-assembling nanoparticles, immunofocusing and immunotargeting, vaccine-elicited and infection-induced immunity, antibody discovery, animal models, and virus-host co-evolution.
Selected Publications
Ellis D, Lederhofer J, Acton OJ, Tsybovsky Y, Kephart S, Yap C, Gillespie RA, Creanga A, Olshefsky A, Stephens T, Pettie D, Murphy M, Sydeman C, Ahlrichs M, Chan S, Borst AJ, Park YJ, Lee KK, Graham BS, Veesler D, King NP, Kanekiyo M. Structure-based design of stabilized recombinant influenza neuraminidase tetramers. Nat Commun. 2022 Apr 5;13(1):1825.
Kanekiyo M, Graham BS. Next-Generation Influenza Vaccines. Cold Spring Harb Perspect Med. 2021 Aug 2;11(8):a038448.
Boyoglu-Barnum S, Ellis D, Gillespie RA, Hutchinson GB, Park YJ, Moin SM, Acton OJ, Ravichandran R, Murphy M, Pettie D, Matheson N, Carter L, Creanga A, Watson MJ, Kephart S, Ataca S, Vaile JR, Ueda G, Crank MC, Stewart L, Lee KK, Guttman M, Baker D, Mascola JR, Veesler D, Graham BS, King NP, Kanekiyo M. Quadrivalent influenza nanoparticle vaccines induce broad protection. Nature. 2021 Apr;592(7855):623-628.
Creanga A, Gillespie RA, Fisher BE, Andrews SF, Lederhofer J, Yap C, Hatch L, Stephens T, Tsybovsky Y, Crank MC, Ledgerwood JE, McDermott AB, Mascola JR, Graham BS, Kanekiyo M. A comprehensive influenza reporter virus panel for high-throughput deep profiling of neutralizing antibodies. Nat Commun. 2021 Mar 19;12(1):1722.
Kanekiyo M, Ellis D, King NP. New Vaccine Design and Delivery Technologies. J Infect Dis. 2019 Apr 8;219(Suppl_1):S88-S96.
Kanekiyo M, Joyce MG, Gillespie RA, Gallagher JR, Andrews SF, Yassine HM, Wheatley AK, Fisher BE, Ambrozak DR, Creanga A, Leung K, Yang ES, Boyoglu-Barnum S, Georgiev IS, Tsybovsky Y, Prabhakaran MS, Andersen H, Kong WP, Baxa U, Zephir KL, Ledgerwood JE, Koup RA, Kwong PD, Harris AK, McDermott AB, Mascola JR, Graham BS. Mosaic nanoparticle display of diverse influenza virus hemagglutinins elicits broad B cell responses. Nat Immunol. 2019 Mar;20(3):362-372.

