Translational Mycology Section
Established in 2009
Peter Williamson, M.D., Ph.D.
Chief, Translational Mycology Section
Provides direct clinical care to patients at NIH Clinical Center

Major Areas of Research
- Pathophysiology and treatment of neuroinflammatory syndromes in refractory fungal infections.
- Genetic susceptibility to infections by Cryptococcus
- Role of RNA biology in susceptibility to human fungal disease
- Granulocyte-monocyte colony stimulating factor (GMCSF) signaling in fungal susceptibility
- Fungal genetic studies of clinical outcome of Cryptococcus in AIDS and solid organ transplant recipients
- International Studies: Use of orally available amphotericin B lipid nano-crystals for treatment of HIV-associated cryptococcal meningitis (Uganda and Ethiopia)
Program Description
The Translational Mycology Section seeks to understand the role of host-pathogen genetics in the outcome of human fungal infections. We use an array of methods from fungal genetics, cell biology, immunology, and population genetics to key aspects of the host-pathogen interface that might facilitate personalized therapeutic interventions.
The laboratory currently focuses on the neurotropic pathogen Cryptococcus which is a major cause of mortality in AIDS as well as in solid-organ transplant recipients and previously healthy individuals. Candida albicans is a major cause of bloodstream infections in the United States.
Biography
Education
M.D., Ph.D., 1987, Boston University, Boston, MA
Dr. Williamson received his M.D./Ph.D. from Boston University in 1987 and completed a residency in internal medicine at Georgetown University before coming to the National Institutes of Health (NIH) for a fellowship in infectious diseases. In 1995, after serving a short stint as chief medical officer, Lalmba Sudan, Dr. Williamson joined the faculty at the University of Illinois at Chicago as an assistant professor of medicine in the section of infectious diseases. After progressing to the rank of professor of medicine, pathology, microbiology, and immunology, Dr. Williamson then returned to NIH to head the Translational Mycology Section in the Laboratory of Clinical Infectious Diseases.
Selected Publications
Hu G, Hauk PJ, Zhang N, Elsegeiny W, Guardia CM, Kullas A, Crosby K, Deterding RR, Schedel M, Reynolds P, Abbott JK, Knight V, Pittaluga S, Raffeld M, Rosenzweig SD, Bonifacino JS, Uzel G, Williamson PR, Gelfand EW. Autophagy-associated immune dysregulation and hyperplasia in a patient with compound heterozygous mutations in ATG9A. Autophagy. 2022 Jul 15:1-14.
Anjum S, Dean O, Kosa P, Magone MT, King KA, Fitzgibbon E, Kim HJ, Zalewski C, Murphy E, Billioux BJ, Chisholm J, Brewer CC, Krieger C, Elsegeiny W, Scott TL, Wang J, Hunsberger S, Bennett JE, Nath A, Marr KA, Bielekova B, Wendler D, Hammoud DA, Williamson P. Outcomes in Previously Healthy Cryptococcal Meningoencephalitis Patients Treated With Pulse Taper Corticosteroids for Post-infectious Inflammatory Syndrome. Clin Infect Dis. 2021 Nov 2;73(9):e2789-e2798.
Lu R, Hollingsworth C, Qiu J, Wang A, Hughes E, Xin X, Konrath KM, Elsegeiny W, Park YD, Atakulu L, Craft JC, Tramont EC, Mannino R, Williamson PR. Efficacy of Oral Encochleated Amphotericin B in a Mouse Model of Cryptococcal Meningoencephalitis. mBio. 2019 May 28;10(3):e00724-19.
Marr KA, Sun Y, Spec A, Lu N, Panackal AA, Bennett JE, Pappas P, Ostrander D, Datta K, Zhang SX, Williamson PR; Cryptococccus infection network cohort (CINCH) study working group. A multicenter, longitudinal cohort study of cryptococcosis in HIV-negative people in the United States. Clin Infect Dis 2019
Park YD, Jarvis JN, Hu G, Davis SE, Qiu J, Zhang N, Hollingsworth C, Loyse A, Gardina PJ, Valyi-Nagy T, Myers TG, Harrison TS, Bicanic T, Williamson PR. Transcriptional Profiling of Patient Isolates Identifies a Novel TOR/Starvation Regulatory Pathway in Cryptococcal Virulence. mBio. 2018 Dec 18;9(6):e02353-18.
Hu G, McQuiston T, Bernard A, Park YD, Qiu J, Vural A, Zhang N, Waterman SR, Blewett NH, Myers TG, Maraia RJ, Kehrl JH, Uzel G, Klionsky DJ, Williamson PR. A conserved mechanism of TOR-dependent RCK-mediated mRNA degradation regulates autophagy. Nat Cell Biol. 2015 Jul;17(7):930-942.
Featured Research
Understanding the Immune Response to Cryptococcus in Healthy People
NIH researchers show that Cryptococcus infection progresses differently in healthy people compared to those with underlying infections like HIV. The work suggests that different therapies should be explored for healthy people who develop the disease.
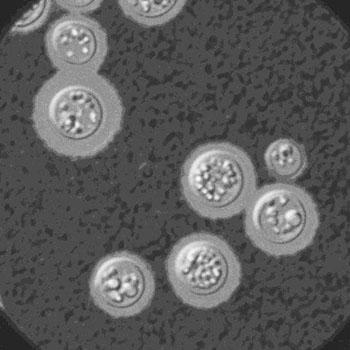
The fungus Cryptococcus neoformans can cause brain inflammation, or meningitis.
Research Group
Cell Biology and Immunology of Fungal Infections


