The Research Technologies Branch (RTB) Rocky Mountain Laboratories (RML) Microscopy Unit provides expertise in both light and electron microscopy related techniques and technologies to support the structural imaging needs of the Division of Intramural Research (DIR) scientists both in Maryland and at the Rocky Mountain Laboratories (RML) in Montana.
The facility provides sample preparation, imaging, and analysis ranging from basic structural studies and immune localization of selected antigens to high resolution and three-dimensional analyses for a wide array of specimens. Recent addition of a focused ion beam scanning electron microscope (FIB-SEM), light microscopes (LM), digital spatial profiling system and expansion of the cryo-EM technologies both at the Bethesda and RML campuses provide greater access for DIR scientists to advanced imaging and spatial profiling technologies.
Major Areas of Support
- 3D Tomography and High-Resolution TEM
- Confocal Microscopy
- Light Microscopy
- Digital Spatial Profiling
- Correlative Light and Electron Microscopy (CLEM)
- Cryo-Electron Microscopy
- Immuno-Electron Microscopy
- Scanning Electron Microscopy (SEM)
- Transmission Electron Microscopy (TEM)
- Focused Ion Beam Scanning Electron Microscopy (FIB-SEM)
Instrumentation
The RML Microscopy Unit has state-of-the-art microscopy equipment, including two scanning electron microscopes, a high-pressure freezer, high-resolution transmission electron microscopes with 3D tomography capabilities, and more. Instruments include from ThermoFisher two 300 kV Titan Krios microscopes with Gatan K3 energy filtered cameras, the 120 kV Tecnai and the Helios G4 focused-ion beam SEM. Hitachi instruments include the H-7800 120 kV TEM and the SU-8000 SEM. Light microscopy facility includes a Zeiss 880 LSM with airyscan.

Titan Krios 300kV TEM enables higher resolution for 2- and 3-D imaging of room-temperature or cryo specimens less than or equal to 1 micron

ThermoFisher Helios 30 kV focused ion beam scanning EM (FIB-SEM) with cryo-stage - provides high resolution topographical imaging for room temperature or cryo-immobilized structures, and the ability to mill through a sample and collect data for 3D volume reconstruction of whole cells

Hitachi SU-8000, a semi-in-lens 30 kV field emission electron microscope with secondary, backscatter, and STEM detector.
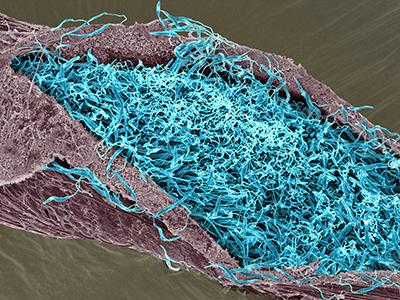
Scanning electron micrograph of leishmania in the midgut of a sand fly.
Hitachi H-7800 120 kilovolt transmission EM (TEM) - provides conventional 2-D and montage high-throughput imaging
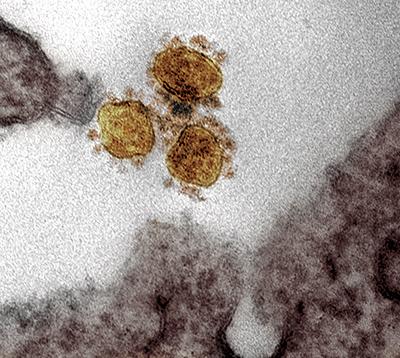
Transmission electron micrograph of SARS-CoV2 in a vero cell.
ThermoFisher FEI/Tecnai 120 kilovolt TEM - offers 3-D tomographic imaging for specimens less than or equal to 250 nanometers
Collaborative Technological Resources
The Microscopy Unit provides assistance with experimental design through consultations, training on core light microscopes, sample processing for electron microscopy (EM), imaging, data collection and analysis for both EM and LM.
Access
Currently, collaborating with RTB is only available to federally funded institutions. Researchers may access all RTB support services by visiting RTB on Inside NIAID (this link is only available to NIAID lab scientists).
Leadership
Beth Fischer, M.A.
Chief, RML Microscopy Unit

Elizabeth Fischer, M.A
Selected Publications
High-resolution structure and strain comparison of infectious mammalian prions.
Kraus A, Hoyt F, Schwartz CL, Hansen B, Artikis E, Hughson AG, Raymond GJ, Race B, Baron GS, Caughey B. Mol Cell. 2021 Nov 4;81(21):4540-4551.e6. doi: 10.1016/j.molcel.2021.08.011. Epub 2021 Aug 25. PMID: 34433091.
Rottlerin inhibits La Crosse virus-induced encephalitis in mice and blocks release of replicating virus from the Golgi body in neurons.
Ojha D, Winkler CW, Leung JM, Woods TA, Chen CZ, Nair V, Taylor K, Yeh CD, Tawa GJ, Larson CL, Zheng W, Haigh CL, Peterson KE. Nat Microbiol. 2021 Nov;6(11):1398-1409. doi: 10.1038/s41564-021-00968-y. Epub 2021 Oct 21. PMID: 34675384.
Disruption of the Golgi Apparatus and Contribution of the Endoplasmic Reticulum to the SARS-CoV-2 Replication Complex.
Hackstadt T, Chiramel AI, Hoyt FH, Williamson BN, Dooley CA, Beare PA, de Wit E, Best SM, Fischer ER. Viruses. 2021 Sep 9;13(9):1798. doi: 10.3390/v13091798. PMID: 34578379; PMCID: PMC8473243.
Cytosolic replication in epithelial cells fuels intestinal expansion and chronic fecal shedding of Salmonella Typhimurium.
Chong A, Cooper KG, Kari L, Nilsson OR, Hillman C, Fleming BA, Wang Q, Nair V, Steele-Mortimer O. Cell Host Microbe. 2021 Jul 14;29(7):1177-1185.e6. doi: 10.1016/j.chom.2021.04.017. Epub 2021 May 26. PMID: 34043959; PMCID: PMC8282707.
The hemifusion structure induced by influenza virus haemagglutinin is determined by physical properties of the target membranes.
Chlanda P, Mekhedov E, Waters H, Schwartz CL, Fischer ER, Ryham RJ, Cohen FS, Blank PS, Zimmerberg J.Nat Microbiol. 2016 Apr 18;1(6):16050. doi: 10.1038/nmicrobiol.2016.50.
Scanning electron microscopy.
Fischer ER, Hansen BT, Nair V, Hoyt FH, Dorward DW. Curr Protoc Microbiol. 2012 May; Chapter 2:Unit 2B.2.