Mouse Genetics and Gene Modification Section
Established in 2015
Jaspal S. Khillan, Ph.D.
Chief, Mouse Genetics and Gene Modification Section

Major Areas of Research
- CRISPR/cas9 mediated genome editing in mouse embryos, ES cells and somatic cells.
- Gene knockout, gene Knock-in, conditional gene knockout for function analysis of genes.
- Generation of transgenic animal models for human genetic disorders and for mechanisms of gene regulation.
- Retinol/Vitamin A signaling in self renewal of stem cells
- Development of embryonic stem (ES) and induced pluripotent stem (iPS) cell lines from different species including mouse and Thicket rat.
- Humanized ACE2 NOD animal models for SARS-CoV-2 for human patients with underlying conditions.
- Cryopreservation of germplasm for long term storage, in vitro fertilization and rederivation of lines.
Program Description
Genome editing via CRISPR cas9 has revolutionized the field of in vivo genome modification which has been used to achieve high editing efficiencies in a wide range of different cell types and embryos to create animal models. A small 20 base guide RNA (gRNA) can locate Cas9 endonuclease to the targeted site which causes the double stranded break (DSB) in the genomic DNA. The DSB is then filled with either non-specific non-homologous end joining (NHEJ), indels, or by more precise homology derived repair (HDR) in the presence of a template with homology sequences. MGGM creates CRISPR cas9 gene edited mouse models for infectious diseases, gene regulation and for specific human mutations as models for human diseases.
The MGGM Core possesses specialized expertise in state-of-the-art technologies for in vivo gene modification in mouse embryos for the production of transgenic, CRISPR mediated gene knockout (KO), gene knock-in (KI) and conditional gene KO mouse models. Gene KO and gene KI mice are also created via gene targeting in ES cells and chimera production. MGGM Core offers genome editing design (guide selection, donor DNA design, and genotyping), in addition to an on- and off-target mutagenesis genotyping service.
MGGM lab is creating humanized animal models for ACE2 enzymes, a receptor for spike protein for corona virus by altering the fifteen mouse amino acids (AA) to human AA as a model for SARS-CoV-2 COVID-19 infection as well as for remedial approaches.
ES cell and iPS cells offer a highly important resource for creation of all types of cells. MGGM is creating ES cell lines from different species including mouse and Thicket rat as model for malaria research. The iPS cells created by reprogramming of somatic cells are pluripotent cells that have complete potential to generate all cell types. MGGM is developing strategies for differentiation of iPS cells into hematopoietic stem cells.
We are also investigating the signaling mechanisms of Vitamin A/retinol in pluripotency and self-renewal of ES and iPS cells. Retinol activates the endogenous machinery to cause over expression of pluripotency specific genes such as Nanog.
The MGGM Core also offers service for cryopreservation of mouse embryos and sperm for long-term storage, rederivation of mouse lines, IVF and mouse colony expansion.
Biography
Education
Ph.D., Punjab University Chandigurh India
Dr. Khillan obtained his Ph.D. from Punjab University Chandigurh India. He has over 25 years of experience gene manipulation in mouse embryos and stem cells. He joined NIAID’s Comparative Medicine Branch (CMB) in 2015 and established the Mouse Genetics and Gene Modification (MGGM) section to provide state of the art technologies of mouse genome modification, including CRISPR/cas9 mediated genome editing, generation of transgenic mouse models, gene targeting in ES cells and iPS cells, germplasm cryopreservation and rederivation of mouse lines. He was the first to establish transgenic technology at NIH. His areas of research include identification of gene that regulate self-renewal of stem cells, cell signaling, development of ES and iPS cell lines from different species, creation of animal models for human genetic disorders, CRISPR/cas9 genome editing in stem cells and somatic cells and generation of humanized animal models for infectious diseases such as SARS Cov-2 and bacterial diseases. He is a recipient of several awards and US patents. He is proficient in three languages including Punjabi, Hindi and English.
Selected Publications
Ernst O, Sun J, Lin B, Banoth B, Dorrington MG, Liang J, Schwarz B, Stromberg KA, Katz S, Vayttaden SJ, Bradfield CJ, Slepushkina N, Rice CM, Buehler E, Khillan JS, McVicar DW, Bosio CM, Bryant CE, Sutterwala FS, Martin SE, Lal-Nag M, Fraser IDC. A genome-wide screen uncovers multiple roles for mitochondrial nucleoside diphosphate kinase D in inflammasome activation. Sci Signal. 2021 Aug;14(694):eabe0387.
Longenecker G, Cho K, Khillan JS, Kulkarni AB. Cryopreservation Protocols for Genetically Engineered Mice. Curr Protoc. 2021 May;1(5):e138.
Kline JM, Heusinkveld LE, Taranto E, Martin CB, Tomasi AG, Hsu IJ, Cho K, Khillan JS, Murphy PM, Pontejo SM. Structural and functional analysis of Ccr1l1, a Rodentia-restricted eosinophil-selective chemokine receptor homologue. J Biol Chem. 2021 Jan-Jun;296:100373.
Mendenhall MA, Liu S, Portley MK, O'Mard D, Fattah R, Szabo R, Bugge TH, Khillan JS, Leppla SH, Moayeri M. Anthrax lethal factor cleaves regulatory subunits of phosphoinositide-3 kinase to contribute to toxin lethality. Nat Microbiol. 2020 Dec;5(12):1464-1471.
Hall B, Cho A, Limaye A, Cho K, Khillan J, Kulkarni AB. Genome Editing in Mice Using CRISPR/Cas9 Technology. Curr Protoc Cell Biol. 2018 Dec;81(1):e57.
Tools & Equipment
- Equipment: High power microscopes, gel electrophoresis imager, embryo microinjection unit., CRISPR gene targeting electroporator.
- Services: CRISPR gene targeting in embryo and in cells, creation of ES cells and iPS cells, cryopreservation of embryo and sperm and rederivation of mouse lines.
- Database: Mouse embryo and sperm cryopreservation database.
Contacts
- Jaspal S. Khillan, Ph.D., Chief
- Kyoungin Cho Ph.D.; Genome editing Expert
- Cheng-Chao Lin Ph.D., Molecular Biologist
- Katina Krasnec Ph.D., Cell Biologist
- Megan Mallett, Embryo Manipulation Expert
- Jessica Edwards, Germplasm Specialist
Services
CRISPR cas9 genome edited mouse models
- Generation of gene knockout mice via microinjection and electroporation
- Generation of knock-in mice using single stranded oligodenucleotides (ssODNTs)
- Generation of gene knock-in mice via double stranded plasmid DNA or long single stranded DNA (lssDNA)
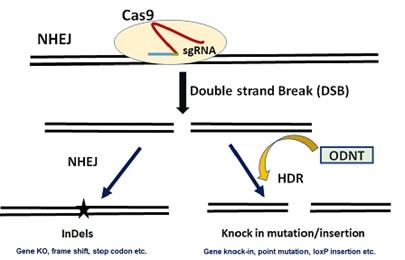
CRISPR/cas9 genome editing. Cas9 protein guided by sgRNA binds the DNA to cause double stranded break (DSB). The break is then filled by random bases via non-homologous end joining (NHEJ) or by homology derived repair in the presence of a template, oligo dinucleotide (ODN) with homology arms.
Transgenic mouse models
- Generation of transgenic mice via traditional DNA injection
- Generation of transgenic mice via Bac injection
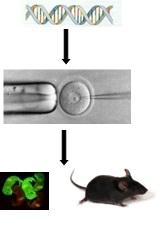
Generation of transgenic animals by DNA microinjection. The genomic DNA containing gene for green florescence protein (GFP), (top), is microinjected into the pronucleus of one cell embryo (middle) to generate pups that exhibit green florescence (bottom).
Gene targeting in ES cells, iPS cells and somatic cells
- Gene targeting in mouse ES cells and chimera production
- CRISPR cas9 genome editing ES cells, iPS cells and somatic cells.
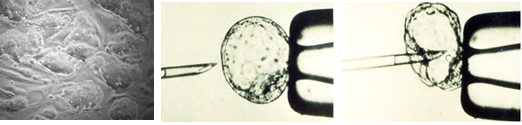
Microinjection of ES cells into Blastocyst. Gene targeted ES cells (left panel) are microinjected into the cavity of expanded blastocyst using very fine needles to create chimeric animals for germline transmission of mutated gene.
Cryopreservation of germplasm and rederivation of lines
- Mouse sperm cryopreservation
- Mouse embryo cryopreservation
- In vitro fertilization (IVF) using mouse sperm
- Rederivation of mouse lines from embryo and sperm
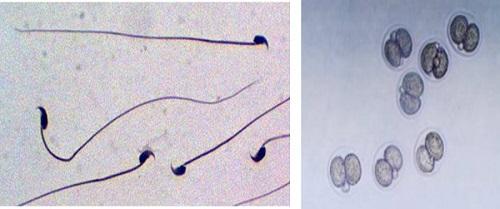
Sperm (left) and embryo cryopreservation (right).
Patents
- Khillan, J, inventors; The United States of America, assignee. A simple and efficient method to generate chimeric animals by co-culture of ES cells and morula stage embryos to prepare transgenic and gene knockout mice. This method does not require elaborate and expensive microinjection setup and is based on one-step co-culture of ES cells with morulas. United States patent US 5,449,620. 12 September 1995.
- Khillan, J, inventors; The United States of America, assignee. An efficient method for production of compound transgenic animals. United States patent US 6,281,408. 28 August 2001.
- Prockop, DJ, Ala-Kokko, L, Khillan, J, Vaudenberg, P, Kontusaari, S, Helminen, H, Olsen, A, Sokolov, B, inventors; The United States of America, assignee. Transgenic mice expressing a mutated collagen gene. United States patent US 5,663,482. 2 September 1997.
- Prockop, DJ, Khillan, J, Li, S, Pereira, R, inventors; The United States of America, assignee. Use of αCOL1A1 mini-gene construct to inhibit collagen synthesis. United States patent US 5,786,341. 28 July 1998.
- Khillan, J, inventors; The United States of America, assignee. Transgenic COL2A1 null mice expressing human COL2A1. United States patent US 6,448,470. 10 September 2002.
Visit the U.S. Patent and Trademark Office for a complete patent listing.
Research Group
We provide CRISPR/cas 9 mediated genome editing in mouse embryos and stem cells, creation of humanized animal models for human genetic and infectious diseases, generation of ES cells and iPS cells from different species, and cryopreservation of sperm and embryos.


