Table of Contents
See the main index of Decision Trees or Research Using Human Subjects.
Graphical Flowchart
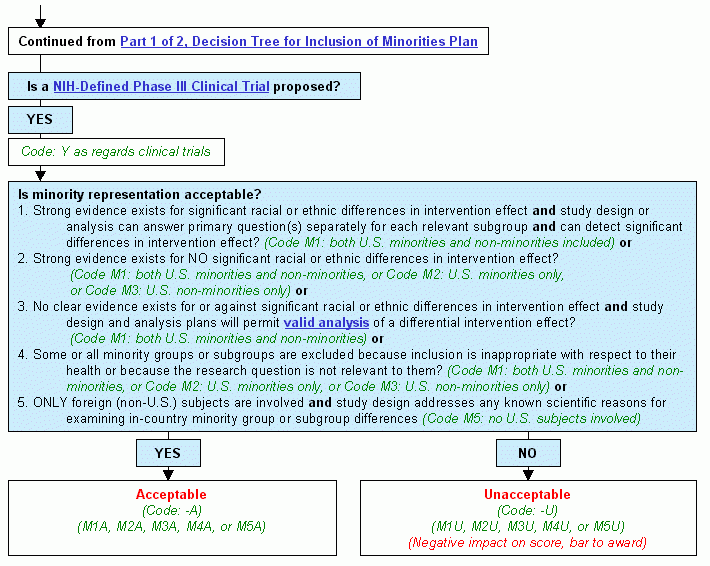
Continued from Decision Tree for Inclusion of Minorities Plan, Part 1 of 2.
Credit:
NIAID
Text Version of Flowchart
Step 1. Is a NIH-Defined Phase III Clinical Trial proposed?
- If no, return to Decision Tree for Inclusion of Minorities Plan, Part 1 of 2.
- If yes:
- Code: Y as regards clinical trials.
- Continue to Step 2.
- Consider these questions:
- Strong evidence exists for significant racial or ethnic differences in intervention effect and study design or analysis can answer primary question(s) separately for each relevant subgroup and can detect significant differences in intervention effect? (Code M1: both U.S. minorities and non-minorities included) or
- Strong evidence exists for NO significant racial or ethnic differences in intervention effect? (Code M1: both U.S. minorities and non-minorities, or Code M2: U.S. minorities only, or Code M3: U.S. non-minorities only) or
- No clear evidence exists for or against significant racial or ethnic differences in intervention effect and study design and analysis plans will permit valid analysis of a differential intervention effect? (Code M1: both U.S. minorities and non-minorities) or
- Some or all minority groups or subgroups are excluded because inclusion is inappropriate with respect to their health or because the research question is not relevant to them? (Code M1: both U.S. minorities and non-minorities, or Code M2: U.S. minorities only, or Code M3: U.S. non-minorities only) or
- Only foreign (non-U.S.) subjects are involved and study design addresses any known scientific reasons for examining in-country minority group or subgroup differences (Code M5: no U.S. subjects involved)
- Is sex representation acceptable?
Summary of Codes
| Minority Representation | Representation is scientifically... | |
|---|---|---|
| Acceptable | Unacceptable (bar to award) | |
| Both minorities and non minorities are included | M1A | M1U |
| minorities only | M2A | M2U |
| non-minorities only | M3A | M3U |
| unknown (cannot be known) | M4A | M4U |
| only foreign (non-U.S subjects) in the study | M5A | M5U |
Clinical Trial Status
- X—Human subjects not involved (not clinical research)
- N—Clinical research, not an NIH-defined Phase III clinical trial
- Y—Clinical research, an NIH-defined Phase III clinical trial

