Table of Contents
See the main index of Decision Trees or Research Using Human Subjects
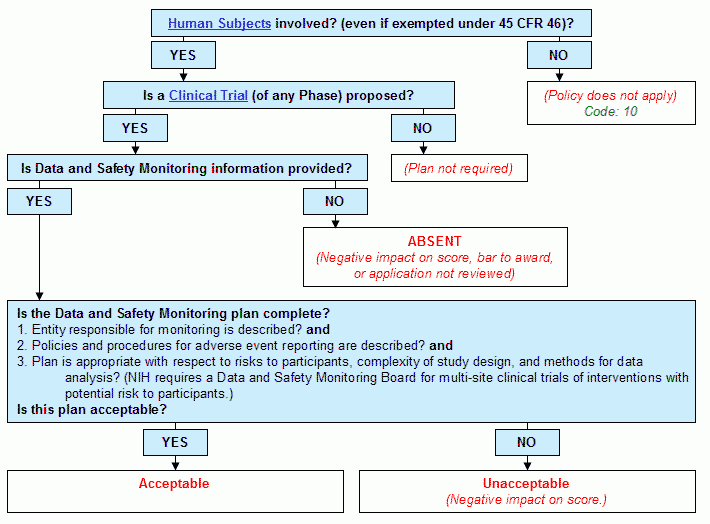
Graphical Flowchart
Credit:
NIAID
Text Version of Flowchart
Step 1. Are human subjects involved? (even if exempted under 45 CFR Part 46)?
- If yes, continue to Step 2.
- If no:
- The policy does not apply.
- Human Subjects Code: 10.
- End.
Step 2. Is a Clinical Trial (of any Phase) proposed?
- If yes:
- Continue to Step 3.
- If no:
- Plan not required.
- End.
Step 3. Is Data and Safety Monitoring information provided?
- If yes:
- Continue to Step 4.
- If no:
- Absent. (Negative impact on score, bar to award, or application not reviewed)
- End.
- Is the Data and Safety Monitoring plan complete?
- Entity responsible for monitoring is described? and
- Policies and procedures for adverse event reporting are described? and
- Plan is appropriate with respect to risks to participants, complexity of study design, and methods for data analysis? (NIH requires a Data and Safety Monitoring Board for multi-site clinical trials of interventions with potential risk to participants.)
- Is this plan acceptable?
- If yes:
- Acceptable.
- End.
- If no:
- Unacceptable
- Negative impact on score.
- End.
- If yes:
Summary of Codes
No specific codes are associated with evaluation as Acceptable or Unacceptable.

