Table of Contents
See the main index of Decision Trees or Research Using Human Subjects.
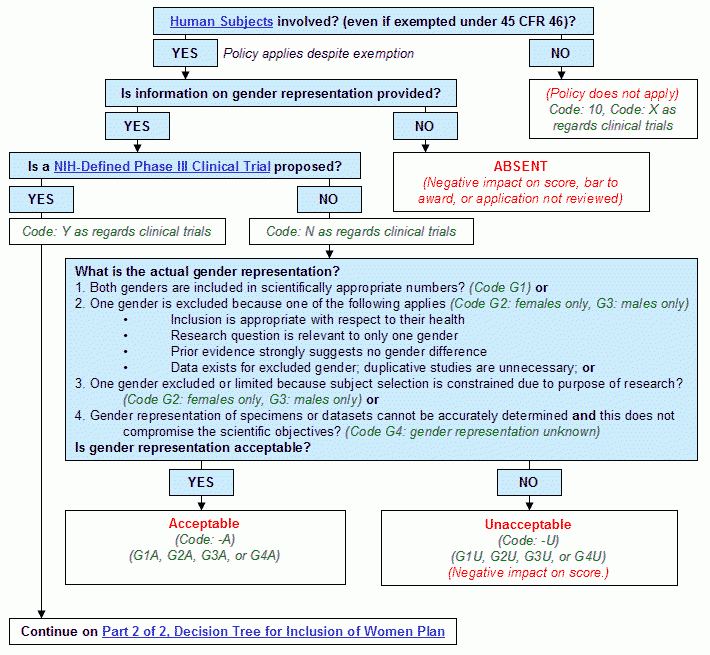
Graphical Flowchart
Credit:
NIAID
Text Version of Flowchart
Step 1. Are human subjects involved? (even if exempted under 45 CFR Part 46)?
- If yes, continue to Step 2.
- If no:
- The policy does not apply.
- Human Subjects Code: 10. X as regards clinical trials.
- End.
Step 2. Is information on gender representation provided?
- If yes, continue to Step 3.
- If no:
- Absent.
- Negative impact on score, bar to award, or application not reviewed.
- End.
Step 3. Is a NIH-defined phase III clinical trial proposed?
- If yes, continue to Decision Tree for Inclusion of Women Plan, Part 2 of 2.
- If no:
- Human Subjects Code: N as regards clinical trials.
- Proceed to Step 4.
- What is the actual gender representation?
- Both genders are included in scientifically appropriate numbers? (Code G1) or
- One gender is excluded because one of the following applies (Code G2: females only, G3: males only)
- Inclusion is appropriate with respect to their health
- Research question is relevant to only one gender
- Prior evidence strongly suggests no gender difference
- Data exists for excluded gender; duplicative studies are unnecessary; or
- One gender excluded or limited because subject selection is constrained due to purpose of research? (Code G2: females only, G3: males only) or
- Gender representation of specimens or datasets cannot be accurately determined and this does not compromise the scientific objectives? (Code G4: gender representation unknown)
- Is gender representation acceptable?
Summary of Codes
| Gender Representation | Representation is scientifically... | |
|---|---|---|
| Acceptable | Unacceptable (bar to award) | |
| Both genders included | G1A | G1U |
| Females only | G2A | G2U |
| Males only | G3A | G3U |
| Unknown (cannot be known) | G4A | G4U |
Clinical Trial Status
- X—Human subjects not involved (not clinical research)
- N—Clinical research, not an NIH-defined Phase III clinical trial
- Y—Clinical research, an NIH-defined Phase III clinical trial

